Copper Catalyzed Oxidative Arylation of Tertiary Carbon Centers
- PMID: 33263410
- PMCID: PMC7885265
- DOI: 10.1021/acs.orglett.0c03581
Copper Catalyzed Oxidative Arylation of Tertiary Carbon Centers
Abstract
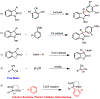
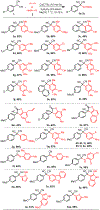
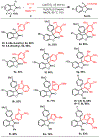
We describe herein a Cu(OTf)2 catalyzed oxidative arylation of a tertiary carbon-containing substrate including aryl malononitriles, 3-aryl benzofuran-2-ones, and 3-aryl oxindoles. In some cases, the nitrile groups of the aryl malononitriles undergo further reactions leading to lactones or imines. These reaction conditions are applicable for a range of arenes, including phenols, anilines, anisoles, and heteroarenes. Mechanistic studies support the formation of a cationic intermediate via a two-electron oxidation.
Figures
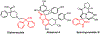
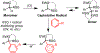
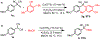
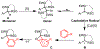
Similar articles
-
Ligand-Promoted Meta-C-H Arylation of Anilines, Phenols, and Heterocycles.J Am Chem Soc. 2016 Jul 27;138(29):9269-76. doi: 10.1021/jacs.6b04966. Epub 2016 Jul 19. J Am Chem Soc. 2016. PMID: 27384126 Free PMC article.
-
TBDPS and Br-TBDPS protecting groups as efficient aryl group donors in Pd-catalyzed arylation of phenols and anilines.J Am Chem Soc. 2009 Aug 12;131(31):10844-5. doi: 10.1021/ja904791y. J Am Chem Soc. 2009. PMID: 19722665 Free PMC article.
-
Aerobic Catalyzed Oxidative Cross-Coupling of N,N-Disubstituted Anilines and Aminonaphthalenes with Phenols and Naphthols.Org Lett. 2020 Mar 6;22(5):1765-1770. doi: 10.1021/acs.orglett.0c00046. Epub 2020 Feb 12. Org Lett. 2020. PMID: 32049541 Free PMC article.
-
Aerobic copper-catalyzed organic reactions.Chem Rev. 2013 Aug 14;113(8):6234-458. doi: 10.1021/cr300527g. Epub 2013 Jun 20. Chem Rev. 2013. PMID: 23786461 Free PMC article. Review. No abstract available.
-
Removal of amino groups from anilines through diazonium salt-based reactions.Org Biomol Chem. 2014 Sep 28;12(36):6965-71. doi: 10.1039/c4ob01286k. Org Biomol Chem. 2014. PMID: 25093920 Review.
Cited by
-
Pd-catalyzed Aerobic Cross-Dehydrogenative Coupling of Catechols with 2-Oxindoles and Benzofuranones: Reactivity Difference Between Monomer and Dimer.Chem Asian J. 2022 Oct 17;17(20):e202200807. doi: 10.1002/asia.202200807. Epub 2022 Sep 22. Chem Asian J. 2022. PMID: 36062560 Free PMC article.
-
Csp2-H functionalization of phenols: an effective access route to valuable materials via Csp2-C bond formation.Chem Sci. 2024 Feb 21;15(11):3831-3871. doi: 10.1039/d4sc00687a. eCollection 2024 Mar 13. Chem Sci. 2024. PMID: 38487228 Free PMC article. Review.
-
Oxindole synthesis via polar-radical crossover of ketene-derived amide enolates in a formal [3 + 2] cycloaddition.Chem Sci. 2022 Mar 9;13(13):3875-3879. doi: 10.1039/d1sc07134c. eCollection 2022 Mar 30. Chem Sci. 2022. PMID: 35432887 Free PMC article.
-
Catalytic Oxidative Coupling of Phenols and Related Compounds.ACS Catal. 2022 Jun 3;12(11):6532-6549. doi: 10.1021/acscatal.2c00318. Epub 2022 May 18. ACS Catal. 2022. PMID: 35928569 Free PMC article.
-
Improved Method for Preparation of 3-(1H-Indol-3-yl)benzofuran-2(3H)-ones.Molecules. 2022 Mar 15;27(6):1902. doi: 10.3390/molecules27061902. Molecules. 2022. PMID: 35335265 Free PMC article.
References
-
- Büschleb M; Dorich S; Hanessian S; Tao D; Schenthal KB; Overman LE Synthetic Strategies toward Natural Products Containing Contiguous Stereogenic Quaternary Carbon Atoms. Angew. Chemie., Int. Ed 2016, 55, 4156–4186. - PMC - PubMed
- Peterson EA; Overman LE Contiguous Stereogenic Quaternary Carbons: A Daunting Challenge in Natural Products Synthesis. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 11943–11948. - PMC - PubMed
-
- Thuy-Boun PS; Villa G; Dang D; Richardson P; Su S; Yu JQ Ligand-Accelerated Ortho -C-H Alkylation of Arylcarboxylic Acids Using Alkyl Boron Reagents. J. Am. Chem. Soc 2013, 135, 17508–17513. - PMC - PubMed
- Li J; Warratz S; Zell D; De Sarkar S; Ishikawa EE; Ackermann L N-Acyl Amino Acid Ligands for Ruthenium(II)-Catalyzed Meta-C-H Tert-Alkylation with Removable Auxiliaries. J. Am. Chem. Soc 2015, 137, 13894–13901. - PubMed
- Paterson AJ; St John-Campbell S; Mahon MF; Press NJ; Frost CG Catalytic Meta-Selective C-H Functionalization to Construct Quaternary Carbon Centres. Chem. Commun 2015, 51, 12807–12810. - PubMed
- Leitch JA; McMullin CL; Paterson AJ; Mahon MF; Bhonoah Y; Frost CG Ruthenium-Catalyzed Para-Selective C−H Alkylation of Aniline Derivatives. Angew. Chemie., Int. Ed 2017, 56, 15131–15135. - PubMed
-
-
For reviews on CDC reactions, see:
- Li C Exploring C - C Bond Formations Beyond. Accounts 2009, 42, 335–344. - PubMed
- Lyons TW; Sanford MS Palladium-Catalyzed Ligand-Directed C - H Functionalization Reactions. Chem. Rev 2010, 110, 1147–1169. - PMC - PubMed
- Wencel-Delord J; Dröge T; Liu F; Glorius F Towards Mild Metal-Catalyzed C-H Bond Activation. Chem. Soc. Rev 2011, 40, 4740–4761. - PubMed
- Kozlowski MC Oxidative Coupling in Complexity Building Transforms. Acc. Chem. Res 2017, 50, 638–643. - PMC - PubMed
-
-
- Bogle KM; Hirst DJ; Dixon DJ An Organocatalytic Oxidative Coupling Strategy for the Direct Synthesis of Arylated Quaternary Stereogenic Centers. Org. Lett 2007, 9, 4901–4904. - PubMed
- Bogle KM; Hirst DJ; Dixon DJ An Oxidative Coupling for the Synthesis of Arylated Quaternary Stereocentres and Its Application in the Total Synthesis of Powelline and Buphanidrine. Tetrahedron, 2010, 66, 6399–6410.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

