Upper and lower airway inflammation in severe asthmatics: a guide for a precision biologic treatment
- PMID: 33263506
- PMCID: PMC7716065
- DOI: 10.1177/1753466620965151
Upper and lower airway inflammation in severe asthmatics: a guide for a precision biologic treatment
Abstract
Background and aims: Severe asthma may require the prescription of one of the biologic drugs currently available, using surrogate markers of airway inflammation (serum IgE levels and allergic sensitization for anti-IgE, or blood eosinophils for anti-IL5/IL5R). Our objective: to assess upper and lower airway inflammation in severe asthmatics divided according to the eligibility criteria for one of the target biologic treatments.
Methods: We selected 91 severe asthmatics, uncontrolled despite high-dose ICS-LABA, and followed for >6 months with optimization of asthma treatment. Patients underwent clinical, functional and biological assessment, including induced sputum and nasal cytology. They were then clustered according to the eligibility criteria for omalizumab or mepolizumab/benralizumab.
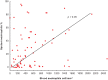
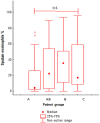
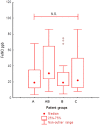
Results: Four clusters were selected: A (eligible for omalizumab, n = 23), AB (both omalizumab and mepolizumab, n = 26), B (mepolizumab, n = 22) and C (non-eligible for both omalizumab and mepolizumab, n = 20). There was no difference among clusters for asthma control (Asthma Control Test and Asthma Control Questionnaire 7), pre-bronchodilator forced expiratory volume in 1 s, serum IgE and fractional exhaled nitric oxide levels. Sputum eosinophils were numerically higher in clusters AB and B, in agreement with the higher levels of blood eosinophils. Allergic rhinitis was more frequent in clusters A and AB, while chronic rhinosinusitis with nasal polyps prevalence increased progressively from A to C. Eosinophils in nasal cytology were higher in clusters AB, B and C.
Conclusion: Eosinophilic upper and lower airway inflammation is present in the large majority of severe asthmatics, independently from the prescription criteria for the currently available biologics, and might suggest the use of anti-IL5/IL5R or anti IL4/13 also in patients without blood eosinophilia.The reviews of this paper are available via the supplemental material section.
Keywords: airway inflammation; biologics; induced sputum; nasal cytology; nasal polyposis; severe asthma.
Conflict of interest statement
Figures
Similar articles
-
Results of personalized biological therapy in patients with chronic rhinosinusitis with nasal polyps and severe uncontrolled bronchial asthma - real-life study.Otolaryngol Pol. 2025 Feb 26;79(2):1-6. doi: 10.5604/01.3001.0054.9674. Otolaryngol Pol. 2025. PMID: 40008473
-
Anti-IgE and Anti-IL5 Biologic Therapy in the Treatment of Nasal Polyposis: A Systematic Review and Meta-analysis.Ann Otol Rhinol Laryngol. 2017 Nov;126(11):739-747. doi: 10.1177/0003489417731782. Epub 2017 Sep 16. Ann Otol Rhinol Laryngol. 2017. PMID: 28918644
-
Switching from omalizumab to mepolizumab: real-life experience from Southern Italy.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620929231. doi: 10.1177/1753466620929231. Ther Adv Respir Dis. 2020. PMID: 32482128 Free PMC article.
-
Association of upper and lower airway eosinophilic inflammation with response to omalizumab in patients with severe asthma.J Asthma. 2020 Jan;57(1):71-78. doi: 10.1080/02770903.2018.1541357. Epub 2018 Nov 29. J Asthma. 2020. PMID: 30489179 Clinical Trial.
-
Biologics in allergic rhinitis.Eur Rev Med Pharmacol Sci. 2023 Oct;27(5 Suppl):43-52. doi: 10.26355/eurrev_202310_34069. Eur Rev Med Pharmacol Sci. 2023. PMID: 37869947 Review.
Cited by
-
Effectiveness of biological therapy in severe asthma: a retrospective real-world study.Croat Med J. 2025 Feb 28;66(1):3-12. doi: 10.3325/cmj.2025.66.3. Croat Med J. 2025. PMID: 40047156 Free PMC article.
-
Nasal cytology as a reliable non-invasive procedure to phenotype patients with type 2 chronic rhinosinusitis with nasal polyps.World Allergy Organ J. 2022 Oct 18;15(11):100700. doi: 10.1016/j.waojou.2022.100700. eCollection 2022 Nov. World Allergy Organ J. 2022. PMID: 36321070 Free PMC article.
-
Switching of biological therapy to dupilumab in comorbid patients with severe asthma and CRSwNP.Eur Arch Otorhinolaryngol. 2024 Jun;281(6):3017-3023. doi: 10.1007/s00405-024-08461-y. Epub 2024 Feb 12. Eur Arch Otorhinolaryngol. 2024. PMID: 38347197 Free PMC article.
-
Personalized Management of Patients with Chronic Rhinosinusitis with Nasal Polyps in Clinical Practice: A Multidisciplinary Consensus Statement.J Pers Med. 2022 May 23;12(5):846. doi: 10.3390/jpm12050846. J Pers Med. 2022. PMID: 35629268 Free PMC article. Review.
-
Advancing Treatment Strategies: A Comprehensive Review of Drug Delivery Innovations for Chronic Inflammatory Respiratory Diseases.Pharmaceutics. 2023 Aug 17;15(8):2151. doi: 10.3390/pharmaceutics15082151. Pharmaceutics. 2023. PMID: 37631365 Free PMC article. Review.
References
-
- Yancey SW, Keene ON, Albers FC, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol 2017; 140: 1509–1518. - PubMed
-
- Robinson D, Humbert M, Buhl R, et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy 2017; 47: 161–175. - PubMed
-
- Dente FL, Latorre M, Novelli F, et al. Can sputum eosinophilia be a constant feature in severe refractory asthmatics? A 3-year longitudinal study. Int Arch Allergy Immunol 2015; 166: 287–290. - PubMed
-
- Amorim MM, Araruna A, Caetano LB, et al. Nasal eosinophilia: an indicator of eosinophilic inflammation in asthma. Clin Exp Allergy 2010; 40: 867–874. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

