Differential Toxicological Outcome of Corn Oil Exposure in Rats and Mice as Assessed by Microbial Composition, Epithelial Permeability, and Ileal Mucosa-Associated Immune Status
- PMID: 33263755
- PMCID: PMC7916742
- DOI: 10.1093/toxsci/kfaa177
Differential Toxicological Outcome of Corn Oil Exposure in Rats and Mice as Assessed by Microbial Composition, Epithelial Permeability, and Ileal Mucosa-Associated Immune Status
Abstract
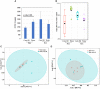
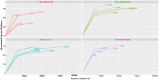
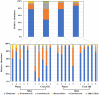
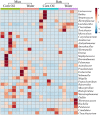
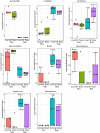
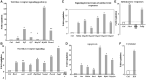
Studies to evaluate the toxicity of xenobiotics on the human gut microbiome and related health effects require a diligent selection of (1) an appropriate animal model to facilitate toxicity assessment in predicting human exposure, and (2) an appropriate non-interfering vehicle for the administration of water insoluble compounds. In biomedical studies with water insoluble xenobiotics, corn oil is one of the most commonly used nonaqueous vehicles. This study evaluated the suitability of corn oil as a vehicle in adult female Sprague Dawley rats and adult CD-1 mice; the rodent models that are often utilized in toxicological studies. We studied the host response in terms of change in the intestinal microbiome and mRNA expression of intestinal permeability and immune response-related genes when water (control) and corn oil (2 ml/kg) were administered as a vehicle through oral gavage. The results showed that the use of corn oil as a vehicle has no adverse impact in rats for either the immune response or the intestinal microbial population. On the other hand, mice treated with corn oil showed changes in bacterial community adhered to the ileum, as well as changes in the mRNA expression of intestinal permeability-related and ileal mucosa-associated immune response genes. Overall, results of this study suggest that the type of rodent species and vehicle used in toxicological risk assessments of xenobiotics studies should be taken into consideration in the experimental setup and study design.
Keywords: corn oil; immune response; intestinal permeability; microbiome; toxicology; xenobiotics.
Published by Oxford University Press on behalf of the Society of Toxicology 2020.
Figures



References
-
- Ajibade A. A., Wang H. Y., Wang R. F. (2013). Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 34, 307–316. - PubMed
-
- Akira S., Uematsu S., Takeuchi O. (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. - PubMed
-
- Andersen A., Daniel L., Mølbak K., Fleischer Michaelsen L.Lauritzen, (2011). Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J. Pediatr. Gastroenterol. Nutr. 53, 303–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

