A Retrospective Study on the Effects of Convalescent Plasma Therapy in 24 Patients Diagnosed with COVID-19 Pneumonia in February and March 2020 at 2 Centers in Wuhan, China
- PMID: 33264276
- PMCID: PMC7720430
- DOI: 10.12659/MSM.928755
A Retrospective Study on the Effects of Convalescent Plasma Therapy in 24 Patients Diagnosed with COVID-19 Pneumonia in February and March 2020 at 2 Centers in Wuhan, China
Abstract
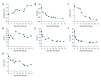
BACKGROUND This retrospective study aimed to describe the effects of convalescent plasma therapy in 24 patients diagnosed with coronavirus disease 2019 (COVID-19) pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during February and March 2020 in Wuhan, China. MATERIAL AND METHODS The confirmation of SARS-CoV-2 infection was made by the reverse transcription-polymerase chain reaction test. We retrospectively analyzed the clinical data and laboratory test reports of patients with severe COVID-19 pneumonia who received a convalescent plasma transfusion. RESULTS A total of 24 patients with COVID-19 pneumonia who were transfused with ABO-compatible convalescent plasma were enrolled in the study. Convalescent plasma transfusion showed an effective clinical outcome in 14 of 24 patients (an effective rate of 58.3%). No patients had an adverse reaction to the transfusion. Compared with before convalescent plasma transfusion, the lymphocyte count after convalescent plasma transfusion increased to a normal level (median: 0.80×10⁹/L vs. 1.12×10⁹/L, P=0.004). Other laboratory indicators such as white blood cells, high-sensitivity C-reactive protein, procalcitonin, alanine aminotransferase, and aspartate transaminase showed a decreasing trend after transfusion. CONCLUSIONS This retrospective observational clinical study showed that convalescent plasma therapy could have beneficial effects on patient outcomes. Recently, regulatory authorization has been given for the use of convalescent plasma therapy, and clinical guidelines have been developed for the collection and use of convalescent plasma and hyperimmune immunoglobulin in patients with COVID-19.
Conflict of interest statement
None.
Figures
Similar articles
-
Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma.Transfusion. 2021 Jun;61(6):1705-1709. doi: 10.1111/trf.16378. Epub 2021 Mar 22. Transfusion. 2021. PMID: 33715160 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial.JAMA. 2020 Aug 4;324(5):460-470. doi: 10.1001/jama.2020.10044. JAMA. 2020. PMID: 32492084 Free PMC article. Clinical Trial.
-
[Effectiveness of convalescent plasma for treatment of coronavirus disease 2019 patients].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020 Nov;32(11):1293-1298. doi: 10.3760/cma.j.cn121430-20200810-00568. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020. PMID: 33463485 Chinese.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
Cited by
-
Passive Immunity Should and Will Work for COVID-19 for Some Patients.Clin Hematol Int. 2021 Apr 16;3(2):47-68. doi: 10.2991/chi.k.210328.001. eCollection 2021 Jun. Clin Hematol Int. 2021. PMID: 34595467 Free PMC article. Review.
-
Current advances in transfusion medicine 2020: A critical review of selected topics by the AABB Clinical Transfusion Medicine Committee.Transfusion. 2021 Sep;61(9):2756-2767. doi: 10.1111/trf.16625. Epub 2021 Aug 22. Transfusion. 2021. PMID: 34423446 Free PMC article. Review.
-
Efficacy and Safety of Blood Derivative Therapy for Patients with COVID-19: A Systematic Review and Meta-Analysis.Transfus Med Hemother. 2022 Apr 25;382(6):1-13. doi: 10.1159/000524125. Transfus Med Hemother. 2022. PMID: 35665313 Free PMC article.
-
COVID-19 Convalescent Plasma Is More than Neutralizing Antibodies: A Narrative Review of Potential Beneficial and Detrimental Co-Factors.Viruses. 2021 Aug 11;13(8):1594. doi: 10.3390/v13081594. Viruses. 2021. PMID: 34452459 Free PMC article. Review.
-
COVID-19: The Disease, the Immunological Challenges, the Treatment with Pharmaceuticals and Low-Dose Ionizing Radiation.Cells. 2021 Aug 27;10(9):2212. doi: 10.3390/cells10092212. Cells. 2021. PMID: 34571861 Free PMC article. Review.
References
-
- World Health Organization. Use of laboratory methods for SARS diagnosis. 2020. https://www.who.int/csr/sars/labmethods/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

