A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19)
- PMID: 33264337
- PMCID: PMC7710068
- DOI: 10.1371/journal.pone.0242763
A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19)
Abstract
Objective: In this study, we evaluated the efficacy of hydroxychloroquine (HCQ) against coronavirus disease 2019 (COVID-19) via a randomized controlled trial (RCT) and a retrospective study.
Methods: Subjects admitted to 11 designated public hospitals in Taiwan between April 1 and May 31, 2020, with COVID-19 diagnosis confirmed by pharyngeal real-time RT-PCR for SARS-CoV-2, were randomized at a 2:1 ratio and stratified by mild or moderate illness. HCQ (400 mg twice for 1 d or HCQ 200 mg twice daily for 6 days) was administered. Both the study and control group received standard of care (SOC). Pharyngeal swabs and sputum were collected every other day. The proportion and time to negative viral PCR were assessed on day 14. In the retrospective study, medical records were reviewed for patients admitted before March 31, 2020.
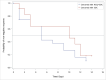
Results: There were 33 and 37 cases in the RCT and retrospective study, respectively. In the RCT, the median times to negative rRT-PCR from randomization to hospital day 14 were 5 days (95% CI; 1, 9 days) and 10 days (95% CI; 2, 12 days) for the HCQ and SOC groups, respectively (p = 0.40). On day 14, 81.0% (17/21) and 75.0% (9/12) of the subjects in the HCQ and SOC groups, respectively, had undetected virus (p = 0.36). In the retrospective study, 12 (42.9%) in the HCQ group and 5 (55.6%) in the control group had negative rRT-PCR results on hospital day 14 (p = 0.70).
Conclusions: Neither study demonstrated that HCQ shortened viral shedding in mild to moderate COVID-19 subjects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. Naming the coronavirus disease (COVID-19) and the viral that caused it [cited 2020 June 20]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica...
-
- World Health Organization. Statement on the second meeting or the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) [cited 2020 June 20]. Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-...
-
- World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID-19 [cited 2020 June 20]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- World Health Organization. Novel coronavirus situation report-151. Geneva, Switzerland [cited 2020 June 20]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

