Auxin response factors (ARFs) differentially regulate rice antiviral immune response against rice dwarf virus
- PMID: 33264360
- PMCID: PMC7735678
- DOI: 10.1371/journal.ppat.1009118
Auxin response factors (ARFs) differentially regulate rice antiviral immune response against rice dwarf virus
Abstract
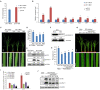
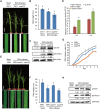
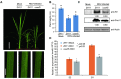
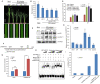
There are 25 auxin response factors (ARFs) in the rice genome, which play critical roles in regulating myriad aspects of plant development, but their role (s) in host antiviral immune defense and the underneath mechanism remain largely unknown. By using the rice-rice dwarf virus (RDV) model system, here we report that auxin signaling enhances rice defense against RDV infection. In turn, RDV infection triggers increased auxin biosynthesis and accumulation in rice, and that treatment with exogenous auxin reduces OsIAA10 protein level, thereby unleashing a group of OsIAA10-interacting OsARFs to mediate downstream antiviral responses. Strikingly, our genetic data showed that loss-of-function mutants of osarf12 or osarf16 exhibit reduced resistance whereas osarf11 mutants display enhanced resistance to RDV. In turn, OsARF12 activates the down-stream OsWRKY13 expression through direct binding to its promoter, loss-of-function mutants of oswrky13 exhibit reduced resistance. These results demonstrated that OsARF 11, 12 and 16 differentially regulate rice antiviral defense. Together with our previous discovery that the viral P2 protein stabilizes OsIAA10 protein via thwarting its interaction with OsTIR1 to enhance viral infection and pathogenesis, our results reveal a novel auxin-IAA10-ARFs-mediated signaling mechanism employed by rice and RDV for defense and counter defense responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

