Translational control of coronaviruses
- PMID: 33264393
- PMCID: PMC7736815
- DOI: 10.1093/nar/gkaa1116
Translational control of coronaviruses
Abstract
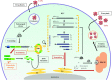
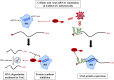
Coronaviruses represent a large family of enveloped RNA viruses that infect a large spectrum of animals. In humans, the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is responsible for the current COVID-19 pandemic and is genetically related to SARS-CoV and Middle East respiratory syndrome-related coronavirus (MERS-CoV), which caused outbreaks in 2002 and 2012, respectively. All viruses described to date entirely rely on the protein synthesis machinery of the host cells to produce proteins required for their replication and spread. As such, virus often need to control the cellular translational apparatus to avoid the first line of the cellular defense intended to limit the viral propagation. Thus, coronaviruses have developed remarkable strategies to hijack the host translational machinery in order to favor viral protein production. In this review, we will describe some of these strategies and will highlight the role of viral proteins and RNAs in this process.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Hamre D., Procknow J.J.. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966; 121:190–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

