Impact of sickle cell disease on patients' daily lives, symptoms reported, and disease management strategies: Results from the international Sickle Cell World Assessment Survey (SWAY)
- PMID: 33264445
- PMCID: PMC8248107
- DOI: 10.1002/ajh.26063
Impact of sickle cell disease on patients' daily lives, symptoms reported, and disease management strategies: Results from the international Sickle Cell World Assessment Survey (SWAY)
Abstract
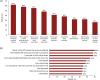
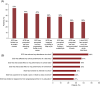
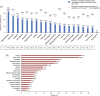
Sickle cell disease (SCD) is a genetic disorder, characterized by hemolytic anemia and vaso-occlusive crises (VOCs). Data on the global SCD impact on quality of life (QoL) from the patient viewpoint are limited. The international Sickle Cell World Assessment Survey (SWAY) aimed to provide insights into patient-reported impact of SCD on QoL. This cross-sectional survey of SCD patients enrolled by healthcare professionals and advocacy groups assessed disease impact on daily life, education and work, symptoms, treatment goals, and disease management. Opinions were captured using a Likert scale of 1-7 for some questions; 5-7 indicated "high severity/impact." Two thousand one hundred and forty five patients (mean age 24.7 years [standard deviation (SD) = 13.1], 39% ≤18 years, 52% female) were surveyed from 16 countries (six geographical regions). A substantial proportion of patients reported that SCD caused a high negative impact on emotions (60%) and school achievement (51%) and a reduction in work hours (53%). A mean of 5.3 VOCs (SD = 6.8) was reported over the 12 months prior to survey (median 3.0 [interquartile range 2.0-6.0]); 24% were managed at home and 76% required healthcare services. Other than VOCs, fatigue was the most commonly reported symptom in the month before survey (65%), graded "high severity" by 67% of patients. Depression and anxiety were reported by 39% and 38% of patients, respectively. The most common patient treatment goal was improving QoL (55%). Findings from SWAY reaffirm that SCD confers a significant burden on patients, epitomized by the high impact on patients' QoL and emotional wellbeing, and the high prevalence of self-reported VOCs and other symptoms.
© 2020 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
I.O. reports consultancy for Novartis and Pfizer; speakers' bureau for Novartis, Terumo, and Global Blood Therapeutics; advisory board participation for Novartis, Pfizer, Acceleron, FORMA Therapeutics, and Global Blood Therapeutics; grants from the Health Resources and Services Administration (HRSA), Patient Centered Outcomes Research Institute (PCORI), and Hemedicus (educational); and Data and Safety Monitoring Board (DSMB) membership for Micella Biopharma. B.A. reports consultancy or membership on an advisory committee for bluebird bio, CRISPR/Vertex, Cyclerion, Forma Therapeutics, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi Genzyme, and Terumo BCT; research funding from Imara and Novartis; and DSMB membership for Global Blood Therapeutics. B.P.D.I. reports unrestricted educational funding from Novartis, bluebird bio, AstraZeneca, Vertex, and Global Blood Therapeutics; and steering committee participation for AstraZeneca, Novartis, and Global Blood Therapeutics. F.E.R. reports research funding from Cyclerion, Novartis, and Pfizer; and advisory board participation for Novartis, Global Blood Therapeutics, and bluebird bio. B.F.‐G. reports employment by the Sickle Cell Disease Association of America. A.N. reports consultancy/expert testimony for Novartis and bluebird bio. C.P.M. reports consultancy for Novartis, Global Blood Therapeutics, bluebird bio, TauTona, Roche, and Emmaus. C.T. reports consultancy/expert testimony for Novartis, Cyclerion, and Global Blood Therapeutics. M.R.A. reports research funding from Novartis, AstraZeneca, and Modus; honoraria from Novartis, Global Blood Therapeutics, and Novo Nordisk; membership on a board of directors/advisory committee for Global Blood Therapeutics, AstraZeneca, Novo Nordisk, and Crispr Therapeutics; and travel support from Amgen. J.‐B.A. reports consultancy/expert testimony and honoraria from Novartis. R.C. reports membership on a board of directors/advisory committee for Novartis, Addmedica, and Global Blood Therapeutics; and consultancy/expert testimony for Novartis. M.dM. reports honoraria and membership on a board of directors/advisory committee for Addmedica, Novartis, bluebird bio, and Vertex. W.J. reports honoraria and consultancy/expert testimony for Novartis. E.N. reports consultancy/expert testimony for Novartis. N.R. is an employee of Novartis Pharma AG. L.D. is an employee of Novartis Pharmaceuticals Corporation. J.J. reports employment by the Sickle Cell Society and honoraria from Novartis. O.R.‐H. and T.B. are employees of Adelphi Real World, which received payment from Novartis Pharmaceuticals as part of this research. S.J. and M.P. report no disclosures.
Figures
Comment in
-
Swaying sickle cell research forward in support of patient reported outcomes.Am J Hematol. 2021 Apr 1;96(4):402-403. doi: 10.1002/ajh.26086. Epub 2021 Jan 22. Am J Hematol. 2021. PMID: 33393092 No abstract available.
References
-
- Ashley‐Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151(9):839‐845. - PubMed
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311‐323. - PubMed
-
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79(1):17‐25. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
