Frontline Science: OX40 agonistic antibody reverses immune suppression and improves survival in sepsis
- PMID: 33264454
- PMCID: PMC7887130
- DOI: 10.1002/JLB.5HI0720-043R
Frontline Science: OX40 agonistic antibody reverses immune suppression and improves survival in sepsis
Abstract
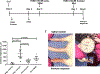
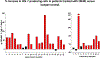
A defining feature of protracted sepsis is development of immunosuppression that is thought to be a major driving force in the morbidity and mortality associated with the syndrome. The immunosuppression that occurs in sepsis is characterized by profound apoptosis-induced depletion of CD4 and CD8 T cells and severely impaired T cell function. OX40, a member of the TNF receptor superfamily, is a positive co-stimulatory molecule expressed on activated T cells. When engaged by OX40 ligand, OX40 stimulates T cell proliferation and shifts the cellular immune phenotype toward TH1 with increased production of cytokines that are essential for control of invading pathogens. The purpose of the present study was to determine if administration of agonistic Ab to OX40 could reverse sepsis-induced immunosuppression, restore T cell function, and improve survival in a clinically relevant animal model of sepsis. The present study demonstrates that OX40 agonistic Ab reversed sepsis-induced impairment of T cell function, increased T cell IFN-γ production, increased the number of immune effector cells, and improved survival in the mouse cecal ligation and puncture model of sepsis. Importantly, OX40 agonistic Ab was not only effective in murine sepsis but also improved T effector cell function in PBMCs from patients with sepsis. The present results provide support for the use of immune adjuvants that target T cell depletion and T cell dysfunction in the therapy of sepsis-induced immunosuppression. In addition to the checkpoint inhibitors anti-PD-1 and anti-PD-L1, OX40 agonistic Ab may be a new therapeutic approach to the treatment of this highly lethal disorder.
Keywords: immunosuppression; lymphocytes, OX40, programmed cell death, sepsis.
©2020 Society for Leukocyte Biology.
Figures





Comment in
-
Reversal of sepsis-induced T cell dysfunction: OX-40 to the rescue?J Leukoc Biol. 2021 Apr;109(4):689-691. doi: 10.1002/JLB.3CE0720-468. Epub 2020 Sep 29. J Leukoc Biol. 2021. PMID: 32991749 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

