Succinyl-CoA Ligase Deficiency in Pro-inflammatory and Tissue-Invasive T Cells
- PMID: 33264602
- PMCID: PMC7755381
- DOI: 10.1016/j.cmet.2020.10.025
Succinyl-CoA Ligase Deficiency in Pro-inflammatory and Tissue-Invasive T Cells
Abstract
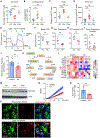
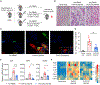
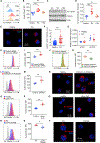
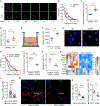
Autoimmune T cells in rheumatoid arthritis (RA) have a defect in mitochondrial oxygen consumption and ATP production. Here, we identified suppression of the GDP-forming β subunit of succinate-CoA ligase (SUCLG2) as an underlying abnormality. SUCLG2-deficient T cells reverted the tricarboxylic acid (TCA) cycle from the oxidative to the reductive direction, accumulated α-ketoglutarate, citrate, and acetyl-CoA (AcCoA), and differentiated into pro-inflammatory effector cells. In AcCoAhi RA T cells, tubulin acetylation stabilized the microtubule cytoskeleton and positioned mitochondria in a perinuclear location, resulting in cellular polarization, uropod formation, T cell migration, and tissue invasion. In the tissue, SUCLG2-deficient T cells functioned as cytokine-producing effector cells and were hyperinflammatory, a defect correctable by replenishing the enzyme. Preventing T cell tubulin acetylation by tubulin acetyltransferase knockdown was sufficient to inhibit synovitis. These data link mitochondrial failure and AcCoA oversupply to autoimmune tissue inflammation.
Keywords: T cell; acetyl-CoA; acetylation; alph-ketoglutarate; autoimmunity; citrate; microtubule; mitochondria; tissue invasion; uropod.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors have declared that no conflict of interest exists.
Figures



Comment in
-
Metabolic defect causes invasive phenotype in RA T cells.Nat Rev Rheumatol. 2021 Mar;17(3):129. doi: 10.1038/s41584-021-00572-8. Nat Rev Rheumatol. 2021. PMID: 33420478 No abstract available.
References
-
- Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, et al. (2016). Memory CD8(+) T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity 44, 1312–1324. - PubMed
-
- Bavister BD (2006). The mitochondrial contribution to stem cell biology. Reprod. Fertil. Dev 18, 829–838. - PubMed
-
- Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M (2014). The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol 15, 536–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

