Self-Organization of Genome Expression from Embryo to Terminal Cell Fate: Single-Cell Statistical Mechanics of Biological Regulation
- PMID: 33265103
- PMCID: PMC7512190
- DOI: 10.3390/e20010013
Self-Organization of Genome Expression from Embryo to Terminal Cell Fate: Single-Cell Statistical Mechanics of Biological Regulation
Abstract
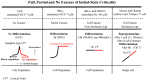
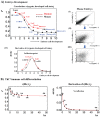
A statistical mechanical mean-field approach to the temporal development of biological regulation provides a phenomenological, but basic description of the dynamical behavior of genome expression in terms of autonomous self-organization with a critical transition (Self-Organized Criticality: SOC). This approach reveals the basis of self-regulation/organization of genome expression, where the extreme complexity of living matter precludes any strict mechanistic approach. The self-organization in SOC involves two critical behaviors: scaling-divergent behavior (genome avalanche) and sandpile-type critical behavior. Genome avalanche patterns-competition between order (scaling) and disorder (divergence) reflect the opposite sequence of events characterizing the self-organization process in embryo development and helper T17 terminal cell differentiation, respectively. On the other hand, the temporal development of sandpile-type criticality (the degree of SOC control) in mouse embryo suggests the existence of an SOC control landscape with a critical transition state (i.e., the erasure of zygote-state criticality). This indicates that a phase transition of the mouse genome before and after reprogramming (immediately after the late 2-cell state) occurs through a dynamical change in a control parameter. This result provides a quantitative open-thermodynamic appreciation of the still largely qualitative notion of the epigenetic landscape. Our results suggest: (i) the existence of coherent waves of condensation/de-condensation in chromatin, which are transmitted across regions of different gene-expression levels along the genome; and (ii) essentially the same critical dynamics we observed for cell-differentiation processes exist in overall RNA expression during embryo development, which is particularly relevant because it gives further proof of SOC control of overall expression as a universal feature.
Keywords: autonomous self-organized criticality; critical transition state; early embryo development; genome avalanche; reprogramming; self-organization; single-cell differentiation; single-cell genome dynamics; statistical thermodynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tsuchiya M., Giuliani A., Yoshikawa K. Single-Cell Reprogramming in Mouse Embryo Development through a Critical Transition State. Entropy. 2017;19:584. doi: 10.3390/e19110584. - DOI
-
- Einstein A., Schilpp P.A. Autobiographical Notes. Open Court Publishing Company; Chicago, IL, USA: 1949.
LinkOut - more resources
Full Text Sources

