Design, Screening, and Testing of Non-Rational Peptide Libraries with Antimicrobial Activity: In Silico and Experimental Approaches
- PMID: 33265897
- PMCID: PMC7759991
- DOI: 10.3390/antibiotics9120854
Design, Screening, and Testing of Non-Rational Peptide Libraries with Antimicrobial Activity: In Silico and Experimental Approaches
Abstract
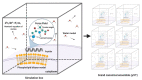
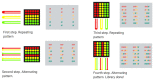
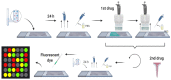
One of the challenges of modern biotechnology is to find new routes to mitigate the resistance to conventional antibiotics. Antimicrobial peptides (AMPs) are an alternative type of biomolecules, naturally present in a wide variety of organisms, with the capacity to overcome the current microorganism resistance threat. Here, we reviewed our recent efforts to develop a new library of non-rationally produced AMPs that relies on bacterial genome inherent diversity and compared it with rationally designed libraries. Our approach is based on a four-stage workflow process that incorporates the interplay of recent developments in four major emerging technologies: artificial intelligence, molecular dynamics, surface-display in microorganisms, and microfluidics. Implementing this framework is challenging because to obtain reliable results, the in silico algorithms to search for candidate AMPs need to overcome issues of the state-of-the-art approaches that limit the possibilities for multi-space data distribution analyses in extremely large databases. We expect to tackle this challenge by using a recently developed classification algorithm based on deep learning models that rely on convolutional layers and gated recurrent units. This will be complemented by carefully tailored molecular dynamics simulations to elucidate specific interactions with lipid bilayers. Candidate AMPs will be recombinantly-expressed on the surface of microorganisms for further screening via different droplet-based microfluidic-based strategies to identify AMPs with the desired lytic abilities. We believe that the proposed approach opens opportunities for searching and screening bioactive peptides for other applications.
Keywords: antibiotic resistance; antimicrobial peptides; deep learning; library screening; microfluidics; molecular dynamics; non-rational; rational.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Stokowski L.A. Antimicrobial Resistance: A Primer. [(accessed on 3 November 2020)]; Available online: https://www.medscape.com/viewarticle/729196.
-
- World Health Organization (WHO) Antimicrobial Resistance. [(accessed on 3 November 2020)]; Available online: https://www.who.int/health-topics/antimicrobial-resistance.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

