Inhibition of EphA2 by Dasatinib Suppresses Radiation-Induced Intestinal Injury
- PMID: 33265912
- PMCID: PMC7730170
- DOI: 10.3390/ijms21239096
Inhibition of EphA2 by Dasatinib Suppresses Radiation-Induced Intestinal Injury
Abstract
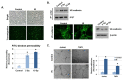
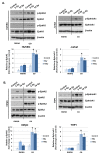
Radiation-induced multiorgan dysfunction is thought to result primarily from damage to the endothelial system, leading to a systemic inflammatory response that is mediated by the recruitment of leukocytes. The Eph-ephrin signaling pathway in the vascular system participates in various disease developmental processes, including cancer and inflammation. In this study, we demonstrate that radiation exposure increased intestinal inflammation via endothelial dysfunction, caused by the radiation-induced activation of EphA2, an Eph receptor tyrosine kinase, and its ligand ephrinA1. Barrier dysfunction in endothelial and epithelial cells was aggravated by vascular endothelial-cadherin disruption and leukocyte adhesion in radiation-induced inflammation both in vitro and in vivo. Among all Eph receptors and their ligands, EphA2 and ephrinA1 were required for barrier destabilization and leukocyte adhesion. Knockdown of EphA2 in endothelial cells reduced radiation-induced endothelial dysfunction. Furthermore, pharmacological inhibition of EphA2-ephrinA1 by the tyrosine kinase inhibitor dasatinib attenuated the loss of vascular integrity and leukocyte adhesion in vitro. Mice administered dasatinib exhibited resistance to radiation injury characterized by reduced barrier leakage and decreased leukocyte infiltration into the intestine. Taken together, these data suggest that dasatinib therapy represents a potential approach for the protection of radiation-mediated intestinal damage by targeting the EphA2-ephrinA1 complex.
Keywords: EphA2; dasatinib; intestinal injury; radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Georgakilas A.G., Pavlopoulou A., Louka M., Nikitaki Z., Vorgias C.E., Bagos P.G., Michalopoulos I. Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Lett. 2015;368:164–172. doi: 10.1016/j.canlet.2015.03.021. - DOI - PubMed
-
- Fliedner T.M., Chao N.J., Bader J.L., Boettger A., Case C., Jr., Chute J., Confer D.L., Ganser A., Gorin N.C., Gourmelon P., et al. Stem cells, multiorgan failure in radiation emergency medical preparedness: A U.S./European Consultation Workshop. Stem Cells. 2009;27:1205–1211. doi: 10.1002/stem.16. - DOI - PMC - PubMed
-
- Fajardo L.F. Is the pathology of radiation injury different in small vs large blood vessels? Cardiovasc. Radiat. Med. 1999;1:108–110. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

