Recent Advances in the Treatment of Patients with Multiple Myeloma
- PMID: 33265952
- PMCID: PMC7761116
- DOI: 10.3390/cancers12123576
Recent Advances in the Treatment of Patients with Multiple Myeloma
Abstract
In the past 20 years, few diseases have seen as great progress in their treatment as multiple myeloma. With the approval of many new drugs and the limited availability of clinical trials comparing head-to-head the different possible combinations, the choice of the best treatments at each stage of the disease becomes complex as well as crucial since multiple myeloma remains incurable. This article presents a general description of the novelties of the whole treatment of multiple myeloma, from induction in the newly diagnosed patient through the role of hematopoietic stem cell transplantation and maintenance treatment until early and late relapses, including a section on recently approved drugs as well as novel drugs and immunotherapy in advanced stages of research, and that will surely play a relevant role in the treatment of this devastating disease in the coming years.
Keywords: autologous stem cell transplantation; car-t cells; consolidation; early relapse; immunotherapy; late relapse; maintenance; multiple myeloma; novel drugs; relapsed refractory multiple myeloma.
Conflict of interest statement
J. de la Rubia: Personal fees from AMGEN, personal fees from BMS, personal fees from Janssen, personal fees from Takeda, personal fees from GSK. Other authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
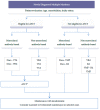
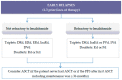

References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.-V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Ferlay J., Colombet M., Soerjomataram I., Dyba T., Randi G., Bettio M., Gavin A., Visser O., Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer Oxf. Engl. 1990. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

