Selective Binding of Cyclodextrins with Leflunomide and Its Pharmacologically Active Metabolite Teriflunomide
- PMID: 33265979
- PMCID: PMC7730839
- DOI: 10.3390/ijms21239102
Selective Binding of Cyclodextrins with Leflunomide and Its Pharmacologically Active Metabolite Teriflunomide
Abstract
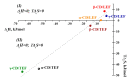
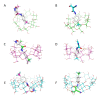
The selectivity of encapsulation of leflunomide and teriflunomide by native α-, β- and γ-cyclodextrins was investigated through 1H NMR and molecular modeling. Thermodynamic analysis revealed the main driving forces involved in the binding. For α-cyclodextrin, the partial encapsulation was obtained while deep penetration was characterized for the other two cyclodextrins, where the remaining polar fragment of the molecule is located outside the macrocyclic cavity. The interactions via hydrogen bonding are responsible for high negative enthalpy and entropy changes accompanying the complexation of cyclodextrins with teriflunomide. These results were in agreement with the molecular modeling calculations, which provide a clearer picture of the involved interactions at the atomic level.
Keywords: complex formation; cyclodextrin; leflunomide; molecular modeling; selectivity; teriflunomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Warnke C., Stüve O., Kieseiera B.C. Teriflunomide for the treatment of multiple sclerosis. Clin. Neurol. Neurosurg. 2013;115S:511–514. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

