Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn's Disease Patients Treated with High-Dose Vitamin Alone or Combined with Infliximab
- PMID: 33266022
- PMCID: PMC7759913
- DOI: 10.3390/nu12123699
Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn's Disease Patients Treated with High-Dose Vitamin Alone or Combined with Infliximab
Abstract
Background: Vitamin D treatment may reduce Crohn's disease (CD) activity by modulating the mucosal immune function. We investigated if high-dose vitamin D +/- infliximab modulated the mucosal cytokine expression in active CD.
Methods: Forty CD patients were randomized into: infliximab + vitamin D; infliximab + placebo-vitamin D; placebo-infliximab + vitamin D or placebo-infliximab + placebo-vitamin D. Infliximab (5 mg/kg) and placebo-infliximab were administered at weeks 0, 2 and 6. Oral vitamin D was administered as bolus 200,000 international units (IU) per week 0 followed by 20,000 IU/day for 7 weeks or placebo. Endoscopy with biopsies was performed at weeks 0 and 7 where endoscopic activity was measured and mucosal mRNA cytokine expression was examined. C-reactive protein (CRP), fecal calprotectin and Harvey-Bradshaw Index (HBI) were measured at weeks 0, 2 and 6.
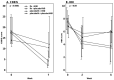
Results: High-dose vitamin D treatment alone and combined with infliximab decreased the IL17A, IFNγ and IL10 expression. High-dose vitamin D alone did not significantly decrease the disease activity, CRP or calprotectin. Combined infliximab and vitamin D treatment was not clinically significantly superior to monotherapy with infliximab.
Conclusions: High-dose vitamin D as monotherapy and combined with infliximab decreases IL17A, IFNγ and IL-10 expression in mucosa within treatment groups. This did not induce a statistically significant decreased disease activity. EudraCT no.2013-000971-34.
Keywords: Crohn’s disease; infliximab; vitamin D treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Colombel J.F., Sandborn W.J., Reinisch W., Mantzaris G.J., Kornbluth A., Rachmilewitz D., Lichtiger S., d’Haens G., Diamond R.H., Broussard D.L., et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

