Activation and Delivery of Tetrazine-Responsive Bioorthogonal Prodrugs
- PMID: 33266075
- PMCID: PMC7731009
- DOI: 10.3390/molecules25235640
Activation and Delivery of Tetrazine-Responsive Bioorthogonal Prodrugs
Abstract
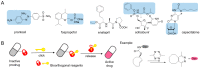
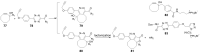
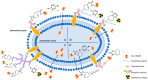
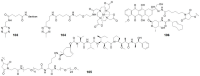
Prodrugs, which remain inert until they are activated under appropriate conditions at the target site, have emerged as an attractive alternative to drugs that lack selectivity and show off-target effects. Prodrugs have traditionally been activated by enzymes, pH or other trigger factors associated with the disease. In recent years, bioorthogonal chemistry has allowed the creation of prodrugs that can be chemically activated with spatio-temporal precision. In particular, tetrazine-responsive bioorthogonal reactions can rapidly activate prodrugs with excellent biocompatibility. This review summarized the recent development of tetrazine bioorthogonal cleavage reaction and great promise for prodrug systems.
Keywords: bioorthogonal reaction; prodrug activation; tetrazine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
An all-in-one tetrazine reagent for cysteine-selective labeling and bioorthogonal activable prodrug construction.Nat Commun. 2024 Apr 2;15(1):2831. doi: 10.1038/s41467-024-47188-6. Nat Commun. 2024. PMID: 38565562 Free PMC article.
-
The Emerging Role of Tetrazines in Drug-Activation Chemistries.Chembiochem. 2019 Apr 1;20(7):872-876. doi: 10.1002/cbic.201800590. Epub 2019 Jan 16. Chembiochem. 2019. PMID: 30394615
-
Tetrazine-Triggered Bioorthogonal Cleavage of trans-Cyclooctene-Caged Phenols Using a Minimal Self-Immolative Linker Strategy.Chembiochem. 2022 Oct 19;23(20):e202200363. doi: 10.1002/cbic.202200363. Epub 2022 Aug 30. Chembiochem. 2022. PMID: 35921044 Free PMC article.
-
Advances in the Delivery, Activation and Therapeutics Applications of Bioorthogonal Prodrugs.Med Res Rev. 2025 May;45(3):887-908. doi: 10.1002/med.22095. Epub 2024 Dec 18. Med Res Rev. 2025. PMID: 39692238 Review.
-
Bioorthogonal Decaging Reactions for Targeted Drug Activation.Chimia (Aarau). 2018 Nov 30;72(11):771-776. doi: 10.2533/chimia.2018.771. Chimia (Aarau). 2018. PMID: 30514419 Review.
Cited by
-
Chemistry-driven translocation of glycosylated proteins in mice.Nat Commun. 2024 Oct 2;15(1):7409. doi: 10.1038/s41467-024-51342-5. Nat Commun. 2024. PMID: 39358337 Free PMC article.
-
Selective Recruitment of Antibodies to Cancer Cells and Immune Cell-mediated Killing via In Situ Click Chemistry.ChemMedChem. 2024 Dec 2;19(23):e202400356. doi: 10.1002/cmdc.202400356. Epub 2024 Oct 26. ChemMedChem. 2024. PMID: 39087480 Free PMC article.
-
Applications of click and click-to-release chemistry in biomaterials to advance skin regeneration.RSC Chem Biol. 2025 Jul 28. doi: 10.1039/d5cb00065c. Online ahead of print. RSC Chem Biol. 2025. PMID: 40880663 Free PMC article. Review.
-
An all-in-one tetrazine reagent for cysteine-selective labeling and bioorthogonal activable prodrug construction.Nat Commun. 2024 Apr 2;15(1):2831. doi: 10.1038/s41467-024-47188-6. Nat Commun. 2024. PMID: 38565562 Free PMC article.
-
Concise Synthesis of Functionalized Cyclobutene Analogues for Bioorthogonal Tetrazine Ligation.Molecules. 2021 Jan 8;26(2):276. doi: 10.3390/molecules26020276. Molecules. 2021. PMID: 33429851 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

