Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts
- PMID: 33266206
- PMCID: PMC7731113
- DOI: 10.3390/ijms21239117
Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts
Abstract
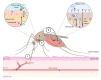
West Nile virus (WNV), like the dengue virus (DENV) and yellow fever virus (YFV), are major arboviruses belonging to the Flavivirus genus. WNV is emerging or endemic in many countries around the world, affecting humans and other vertebrates. Since 1999, it has been considered to be a major public and veterinary health problem, causing diverse pathologies, ranging from a mild febrile state to severe neurological damage and death. WNV is transmitted in a bird-mosquito-bird cycle, and can occasionally infect humans and horses, both highly susceptible to the virus but considered dead-end hosts. Many studies have investigated the molecular determinants of WNV virulence, mainly with the ultimate objective of guiding vaccine development. Several vaccines are used in horses in different parts of the world, but there are no licensed WNV vaccines for humans, suggesting the need for greater understanding of the molecular determinants of virulence and antigenicity in different hosts. Owing to technical and economic considerations, WNV virulence factors have essentially been studied in rodent models, and the results cannot always be transported to mosquito vectors or to avian hosts. In this review, the known molecular determinants of WNV virulence, according to invertebrate (mosquitoes) or vertebrate hosts (mammalian and avian), are presented and discussed. This overview will highlight the differences and similarities found between WNV hosts and models, to provide a foundation for the prediction and anticipation of WNV re-emergence and its risk of global spread.
Keywords: West Nile virus; molecular determinants; vertebrate and invertebrate hosts; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The Immune Responses of the Animal Hosts of West Nile Virus: A Comparison of Insects, Birds, and Mammals.Front Cell Infect Microbiol. 2018 Apr 3;8:96. doi: 10.3389/fcimb.2018.00096. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29666784 Free PMC article. Review.
-
West Nile virus in India: An update on its genetic lineages.J Vector Borne Dis. 2023 Jul-Sep;60(3):225-237. doi: 10.4103/0972-9062.374039. J Vector Borne Dis. 2023. PMID: 37843232 Review.
-
Autochthonous Transmission of West Nile Virus by a New Vector in Iran, Vector-Host Interaction Modeling and Virulence Gene Determinants.Viruses. 2020 Dec 16;12(12):1449. doi: 10.3390/v12121449. Viruses. 2020. PMID: 33339336 Free PMC article.
-
Retrospective review and current knowledge on the occurrence of West Nile virus in mosquito vectors, reservoirs and hosts in Slovakia (Central Europe).Acta Virol. 2020;64(2):187-200. doi: 10.4149/av_2020_209. Acta Virol. 2020. PMID: 32551787 Review.
-
Noncoding Subgenomic Flavivirus RNA Is Processed by the Mosquito RNA Interference Machinery and Determines West Nile Virus Transmission by Culex pipiens Mosquitoes.J Virol. 2016 Oct 28;90(22):10145-10159. doi: 10.1128/JVI.00930-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581979 Free PMC article.
Cited by
-
Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections.Viruses. 2023 Oct 31;15(11):2200. doi: 10.3390/v15112200. Viruses. 2023. PMID: 38005878 Free PMC article. Review.
-
First Detection of West Nile Virus by Nasopharyngeal Swab, Followed by Phylogenetic Analysis.Pathogens. 2024 Nov 20;13(11):1023. doi: 10.3390/pathogens13111023. Pathogens. 2024. PMID: 39599576 Free PMC article.
-
Pathogenesis of Two Western Mediterranean West Nile Virus Lineage 1 Isolates in Experimentally Infected Red-Legged Partridges (Alectoris rufa).Pathogens. 2021 Jun 13;10(6):748. doi: 10.3390/pathogens10060748. Pathogens. 2021. PMID: 34199167 Free PMC article.
-
First Detection of West Nile Virus (WNV) Lineage 2 in Mosquitoes in the Republic of Kosovo.Transbound Emerg Dis. 2025 Jun 24;2025:3208806. doi: 10.1155/tbed/3208806. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40599430 Free PMC article.
-
Continuous and Dynamic Circulation of West Nile Virus in Mosquito Populations in Bucharest Area, Romania, 2017-2023.Microorganisms. 2024 Oct 17;12(10):2080. doi: 10.3390/microorganisms12102080. Microorganisms. 2024. PMID: 39458389 Free PMC article.
References
-
- Smithburn K.C., Hugues T.P., Burke A.W., Paul J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. Hyg. 1940;s1-20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

