Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence
- PMID: 33266246
- PMCID: PMC7729917
- DOI: 10.3390/ijms21239130
Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence
Abstract
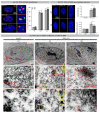
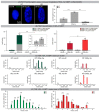
Irreparable double-strand breaks (DSBs) in response to ionizing radiation (IR) trigger prolonged DNA damage response (DDR) and induce premature senescence. Profound chromatin reorganization with formation of senescence-associated heterochromatin foci (SAHF) is an essential epigenetic mechanism for controlling the senescence-associated secretory phenotype (SASP). To decipher molecular mechanisms provoking continuous DDR leading to premature senescence, radiation-induced DSBs (53BP1-foci) and dynamics of histone variant H2A.J incorporation were analyzed together with chromatin re-modeling in human fibroblasts after IR exposure. High-resolution imaging by transmission electron microscopy revealed that persisting 53BP1-foci developed into DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS), consistently located at the periphery of SAHFs. Quantitative immunogold-analysis by electron microscopy revealed that H2A.J, steadily co-localizing with 53BP1, is increasingly incorporated into DNA-SCARS during senescence progression. Strikingly, shRNA-mediated H2A.J depletion in fibroblasts modified senescence-associated chromatin re-structuring and abolished SASP, thereby shutting down the production of inflammatory mediators. These findings provide mechanistic insights into biological phenomena of SASP and suggest that H2A.J inhibition could ablate SASP, without affecting the senescence-associated growth arrest.
Keywords: DNA-SCARS; histone variant H2A.J; radiation-induced senescence; senescence-associated heterochromatin foci (SAHF); senescence-associated secretory phenotype (SASP); transmission electron microscopy (TEM).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




Similar articles
-
Role of Histone Variant H2A.J in Fine-Tuning Chromatin Organization for the Establishment of Ionizing Radiation-Induced Senescence.Cells. 2023 Mar 16;12(6):916. doi: 10.3390/cells12060916. Cells. 2023. PMID: 36980257 Free PMC article.
-
Studies on Human Cultured Fibroblasts and Cutaneous Squamous Cell Carcinomas Suggest That Overexpression of Histone Variant H2A.J Promotes Radioresistance and Oncogenic Transformation.Genes (Basel). 2024 Jun 27;15(7):851. doi: 10.3390/genes15070851. Genes (Basel). 2024. PMID: 39062630 Free PMC article.
-
Immunomodulatory Effects of Histone Variant H2A.J in Ionizing Radiation Dermatitis.Int J Radiat Oncol Biol Phys. 2024 Mar 1;118(3):801-816. doi: 10.1016/j.ijrobp.2023.09.022. Epub 2023 Sep 25. Int J Radiat Oncol Biol Phys. 2024. PMID: 37758068
-
Alteration in the chromatin landscape during the DNA damage response: Continuous rotation of the gear driving cellular senescence and aging.DNA Repair (Amst). 2023 Nov;131:103572. doi: 10.1016/j.dnarep.2023.103572. Epub 2023 Sep 17. DNA Repair (Amst). 2023. PMID: 37742405 Review.
-
Chromatin maintenance and dynamics in senescence: a spotlight on SAHF formation and the epigenome of senescent cells.Chromosoma. 2014 Oct;123(5):423-36. doi: 10.1007/s00412-014-0469-6. Epub 2014 May 27. Chromosoma. 2014. PMID: 24861957 Review.
Cited by
-
Neuronal DNA double-strand breaks lead to genome structural variations and 3D genome disruption in neurodegeneration.Cell. 2023 Sep 28;186(20):4404-4421.e20. doi: 10.1016/j.cell.2023.08.038. Cell. 2023. PMID: 37774679 Free PMC article.
-
Role of Histone Variant H2A.J in Fine-Tuning Chromatin Organization for the Establishment of Ionizing Radiation-Induced Senescence.Cells. 2023 Mar 16;12(6):916. doi: 10.3390/cells12060916. Cells. 2023. PMID: 36980257 Free PMC article.
-
Translating genetic findings to epigenetics: identifying the mechanisms associated with aging after high-radiation exposure on earth and in space.Front Public Health. 2024 Mar 22;12:1333222. doi: 10.3389/fpubh.2024.1333222. eCollection 2024. Front Public Health. 2024. PMID: 38584916 Free PMC article.
-
Oversecretion of CCL3 by Irradiation-Induced Senescent Osteocytes Mediates Bone Homeostasis Imbalance.Cells. 2025 Feb 10;14(4):249. doi: 10.3390/cells14040249. Cells. 2025. PMID: 39996722 Free PMC article.
-
Effects of High-Linear-Energy-Transfer Heavy Ion Radiation on Intestinal Stem Cells: Implications for Gut Health and Tumorigenesis.Cancers (Basel). 2024 Oct 4;16(19):3392. doi: 10.3390/cancers16193392. Cancers (Basel). 2024. PMID: 39410012 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

