Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe
- PMID: 33266306
- PMCID: PMC7731157
- DOI: 10.3390/ijms21239140
Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe
Abstract
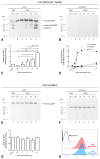
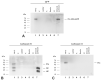
The significance of cysteine cathepsins for the liberation of thyroid hormones from the precursor thyroglobulin was previously shown by in vivo and in vitro studies. Cathepsin L is most important for thyroglobulin processing in mice. The present study aims at specifying the possible contribution of its closest relative, cysteine cathepsin L2/V, to thyroid function. Immunofluorescence analysis on normal human thyroid tissue revealed its predominant localization at the apical plasma membrane of thyrocytes and within the follicle lumen, indicating the secretion of cathepsin V and extracellular tasks rather than its acting within endo-lysosomes. To explore the trafficking pathways of cathepsin V in more detail, a chimeric protein consisting of human cathepsin V tagged with green fluorescent protein (GFP) was stably expressed in the Nthy-ori 3-1 thyroid epithelial cell line. Colocalization studies with compartment-specific markers and analyses of post-translational modifications revealed that the chimeric protein was sorted into the lumen of the endoplasmic reticulum and subsequently transported to the Golgi apparatus, while being N-glycosylated. Immunoblotting showed that the chimeric protein reached endo-lysosomes and it became secreted from the transduced cells. Astonishingly, thyroid stimulating hormone (TSH)-induced secretion of GFP-tagged cathepsin V occurred as the proform, suggesting that TSH upregulates its transport to the plasma membrane before it reaches endo-lysosomes for maturation. The proform of cathepsin V was found to be reactive with the activity-based probe DCG-04, suggesting that it possesses catalytic activity. We propose that TSH-stimulated secretion of procathepsin V is the default pathway in the thyroid to enable its contribution to thyroglobulin processing by extracellular means.
Keywords: cysteine cathepsins; green fluorescent protein tagging; protein trafficking; secretion; thyroid epithelial cells; thyroid stimulating hormone.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Investigations on Primary Cilia of Nthy-ori 3-1 Cells upon Cysteine Cathepsin Inhibition or Thyrotropin Stimulation.Int J Mol Sci. 2023 May 26;24(11):9292. doi: 10.3390/ijms24119292. Int J Mol Sci. 2023. PMID: 37298246 Free PMC article.
-
Significance of nuclear cathepsin V in normal thyroid epithelial and carcinoma cells.Biochim Biophys Acta Mol Cell Res. 2020 Dec;1867(12):118846. doi: 10.1016/j.bbamcr.2020.118846. Epub 2020 Sep 7. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32910988
-
The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice.Int J Mol Sci. 2021 May 28;22(11):5776. doi: 10.3390/ijms22115776. Int J Mol Sci. 2021. PMID: 34071318 Free PMC article.
-
Auto-Regulation of the Thyroid Gland Beyond Classical Pathways.Exp Clin Endocrinol Diabetes. 2020 Jun;128(6-07):437-445. doi: 10.1055/a-1080-2969. Epub 2020 Feb 19. Exp Clin Endocrinol Diabetes. 2020. PMID: 32074633 Review.
-
Cysteine proteinases mediate extracellular prohormone processing in the thyroid.Biol Chem. 2001 May;382(5):717-25. doi: 10.1515/BC.2001.087. Biol Chem. 2001. PMID: 11517924 Review.
Cited by
-
Investigations on Primary Cilia of Nthy-ori 3-1 Cells upon Cysteine Cathepsin Inhibition or Thyrotropin Stimulation.Int J Mol Sci. 2023 May 26;24(11):9292. doi: 10.3390/ijms24119292. Int J Mol Sci. 2023. PMID: 37298246 Free PMC article.
-
Cathepsin V regulates cell cycle progression and histone stability in the nucleus of breast cancer cells.Front Pharmacol. 2023 Nov 6;14:1271435. doi: 10.3389/fphar.2023.1271435. eCollection 2023. Front Pharmacol. 2023. PMID: 38026973 Free PMC article.
-
New inhibitors of cathepsin V impair tumor cell proliferation and elastin degradation and increase immune cell cytotoxicity.Comput Struct Biotechnol J. 2022 Aug 28;20:4667-4687. doi: 10.1016/j.csbj.2022.08.046. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36147668 Free PMC article.
References
-
- Adachi W., Kawamoto S., Ohno I., Nishida K., Kinoshita S., Matsubara K., Okubo K. Isolation and characterization of human cathepsin V: A major proteinase in corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1998;39:1789–1796. - PubMed
-
- Santamaría I., Velasco G., Cazorla M., Fueyo A., Campo E., López-Otín C. Cathepsin L2, a novel human cysteine proteinase produced by breast and colorectal carcinomas. Cancer Res. 1998;58:1624–1630. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

