Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications
- PMID: 33266394
- PMCID: PMC7700471
- DOI: 10.3390/biomedicines8110527
Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications
Abstract
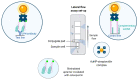
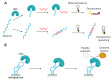
Nucleic acid aptamers capable of affine and specific binding to their molecular targets have now established themselves as a very promising alternative to monoclonal antibodies for diagnostic and therapeutic applications. Although the main focus in aptamers' research and development for biomedicine is made on cardiovascular, infectious, and malignant diseases, the use of aptamers as therapeutic or diagnostic tools in the context of rheumatic diseases is no less important. In this review, we consider the main features of aptamers that make them valuable molecular tools for rheumatologists, and summarize the studies on the selection and application of aptamers for protein biomarkers associated with rheumatic diseases. We discuss the progress in the development of aptamer-based diagnostic assays and targeted therapeutics for rheumatic disorders, future prospects in the field, and issues that have yet to be addressed.
Keywords: aptamer therapeutics; aptamers; aptasensors; protein biomarkers; rheumatic diseases.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

