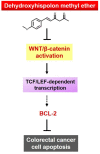
Dehydroxyhispolon Methyl Ether, A Hispolon Derivative, Inhibits WNT/β-Catenin Signaling to Elicit Human Colorectal Carcinoma Cell Apoptosis
- PMID: 33266494
- PMCID: PMC7700694
- DOI: 10.3390/ijms21228839
Dehydroxyhispolon Methyl Ether, A Hispolon Derivative, Inhibits WNT/β-Catenin Signaling to Elicit Human Colorectal Carcinoma Cell Apoptosis
Abstract
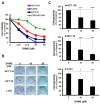
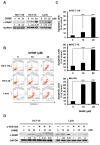
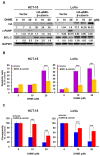
Colorectal cancer (CRC) is the fourth leading cause of cancer mortality worldwide. Aberrant activation of WNT/β-catenin signaling present in the vast majority of CRC cases is indispensable for CRC initiation and progression, and thus is a promising target for CRC therapeutics. Hispolon is a fungal-derived polyphenol with a pronounced anticancer effect. Several hispolon derivatives, including dehydroxyhispolon methyl ether (DHME), have been chemically synthesized for developing lead molecules with stronger anticancer activity. Herein, a DHME-elicited anti-CRC effect with the underlying mechanism is reported for the first time. Specifically, DHME was found to be more cytotoxic than hispolon against a panel of human CRC cell lines, while exerting limited toxicity to normal human colon cell line CCD 841 CoN. Additionally, the cytotoxic effect of DHME appeared to rely on inducing apoptosis. This notion was evidenced by DHME-elicited upregulation of poly (ADP-ribose) polymerase (PARP) cleavage and a cell population positively stained by annexin V, alongside the downregulation of antiapoptotic B-cell lymphoma 2 (BCL-2), whereas the blockade of apoptosis by the pan-caspase inhibitor z-VAD-fmk attenuated DHME-induced cytotoxicity. Further mechanistic inquiry revealed the inhibitory action of DHME on β-catenin-mediated, T-cell factor (TCF)-dependent transcription activity, suggesting that DHME thwarted the aberrantly active WNT/β-catenin signaling in CRC cells. Notably, ectopic expression of a dominant-active β-catenin mutant (∆N90-β-catenin) abolished DHME-induced apoptosis while also restoring BCL-2 expression. Collectively, we identified DHME as a selective proapoptotic agent against CRC cells, exerting more potent cytotoxicity than hispolon, and provoking CRC cell apoptosis via suppression of the WNT/β-catenin signaling axis.
Keywords: Phellinus linteus; WNT/β-catenin; colorectal cancer; dehydroxyhispolon methyl ether; hispolon; hispolon derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jackstadt R., Hodder M.C., Sansom O.J. WNT and β-catenin in cancer: Genes and therapy. Annu. Rev. Cancer Biol. 2020;4:177–196. doi: 10.1146/annurev-cancerbio-030419-033628. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

