Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons
- PMID: 33267610
- PMCID: PMC7902479
- DOI: 10.1161/CIRCULATIONAHA.120.047987
Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons
Abstract
Background: In the ISCHEMIA trial (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches), an initial invasive strategy did not significantly reduce rates of cardiovascular events or all-cause mortality in comparison with a conservative strategy in patients with stable ischemic heart disease and moderate/severe myocardial ischemia. The most frequent component of composite cardiovascular end points was myocardial infarction (MI).
Methods: ISCHEMIA prespecified that the primary and major secondary composite end points of the trial be analyzed using 2 MI definitions. For procedural MI, the primary MI definition used creatine kinase-MB as the preferred biomarker, whereas the secondary definition used cardiac troponin. Procedural thresholds were >5 times the upper reference level for percutaneous coronary intervention and >10 times for coronary artery bypass grafting. Procedural MI definitions included (1) a category of elevated biomarker only events with much higher biomarker thresholds, (2) new ST-segment depression of ≥1 mm for the primary and ≥0.5 mm for the secondary definition, and (3) new coronary dissections >National Heart, Lung, and Blood Institute grade 3. We compared MI type, frequency, and prognosis by treatment assignment using both MI definitions.
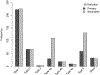
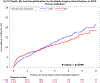
Results: Procedural MIs accounted for 20.1% of all MI events with the primary definition and 40.6% of all MI events with the secondary definition. Four-year MI rates in patients undergoing revascularization were more frequent with the invasive versus conservative strategy using the primary (2.7% versus 1.1%; adjusted hazard ratio [HR], 2.98 [95% CI, 1.87-4.73]) and secondary (8.2% versus 2.0%; adjusted HR, 5.04 [95% CI, 3.64-6.97]) MI definitions. Type 1 MIs were less frequent with the invasive versus conservative strategy using the primary (3.40% versus 6.89%; adjusted HR, 0.53 [95% CI, 0.41-0.69]; P<0.0001) and secondary (3.48% versus 6.89%; adjusted HR, 0.53 [95% CI, 0.41-0.69]; P<0.0001) definitions. The risk of subsequent cardiovascular death was higher after a type 1 MI than after no MI using the primary (adjusted HR, 3.38 [95% CI, 2.03-5.61]; P<0.001) or secondary MI definition (adjusted HR, 3.52 [2.11-5.88]; P<0.001).
Conclusions: In ISCHEMIA, type 1 MI events using the primary and secondary definitions during 5-year follow-up were more frequent with an initial conservative strategy and associated with subsequent cardiovascular death. Procedural MI rates were greater in the invasive strategy and with the use of the secondary MI definition. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01471522.
Keywords: catheterization; drug therapy; myocardial infarction; myocardial ischemia; myocardial revascularization.
Conflict of interest statement
Disclosure Statements
Dr. Bernard R. Chaitman reports grants from National Heart, Lung and Blood Institute during the conduct of the study, personal fees from Merck, NovoNordisk, Sanofi, Lilly, Johnson and Johnson, Daiichi Sankyo, Tricida, Relypsa, Imbria, and Xylocor outside the submitted work;
Dr. Karen P. Alexander reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Derek Cyr reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Jeffrey S. Berger reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Harmony Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from Siemens, non-financial support from BioTelemetry, outside the submitted work;
Dr. Sripal Bangalore repots grants from National Heart, Lung, and Blood Institute during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from Biotronik, personal fees from Pfizer, personal fees from Amgen, personal fees from Reata, outside the submitted work;.
Dr. William E. Boden reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, personal fees from Janssen, outside the submitted work
Dr. Lopes reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; other from Bayer, other from Boehringer Ingleheim, grants and other from Bristol-Myers Squibb, other from Daiichi Sankyo, grants and other from Glaxo Smith Kline, grants and other from Medtronic, other from Merck, grants and other from Pfizer, other from Portola, grants and other from Sanofi, outside the submitted work;.
Dr. Marcin Demkow reports grants from National Heart, Lung and Blood Institute during the conduct of the study and receives proctoring honoraria from ABBOTT, EDWARDS, BOSTON and MEDTRONIC
Dr. Gian Piero Perna reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Robert K. Riezebos reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Edward O. McFalls reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Subhash Banerjee reports grants from National Heart, Lung and Blood Institute during the conduct of the study; reports consulting honoraria from Medtronic, Astra Zeneca, Livmor Inc.; Institutional research grants: Boston Scientific Corp., Chiesi
Dr. Akshay Bagai reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Gilbert Gosselin reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Sean M. O’Brien reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Frank W. Rockhold reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; grants and personal fees from Janssen, personal fees from Merck HeathCare KGaA, personal fees from Merck Research Labs, personal fees from Novo Nordisk, personal fees from KLSMC, personal fees from Aldeyra, personal fees from Rhythm , personal fees from Phathom, grants and personal fees from AstraZeneca, personal fees from Complexa, grants and personal fees from Eidos, other from Athira, other from Spencer Healthcare, outside the submitted work;
Dr. David D. Waters reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Kristian A. Thygesen reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Gregg W. Stone reports grants and personal fees from National Heart, Lung, and Blood Institute , during the conduct of the study; personal fees from Terumo, personal fees from Amaranth, personal fees from Shockwave, personal fees and other from Valfix, personal fees from TherOx, personal fees from Reva, personal fees from Vascular Dynamics, personal fees from Robocath, personal fees from HeartFlow, personal fees from Gore, personal fees from Ablative Solutions, personal fees from Matrizyme, personal fees from Miracor, personal fees from Neovasc, personal fees from V-wave, personal fees from Abiomed, personal fees from Claret, personal fees from Sirtex, personal fees and other from Ancora, personal fees and other from Qool Therapeutics, other from Cagent, other from Applied Therapeutics, other from Biostar family of funds, other from MedFocus family of funds, personal fees and other from SpectraWave, personal fees from MAIA Pharmaceuticals, personal fees and other from Orchestra Biomed, other from Aria, personal fees from Vectorious, other from Cardiac Success, outside the submitted work; .
Dr Harvey D. White reports grants from National Heart, Lung and Blood Institute during the conduct of the study; reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi-Aventis and Regeneron Pharmaceuticals, for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly, for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals, , for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study (A Study to Evaluate the Effect of Long-term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc, for the dal-GenE study (Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc, for the AEGIS-II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd, and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid [ETC-1002] or Placebo) from Esperion Therapeutics Inc. He was on the Advisory Board for Genentech, Inc. and received lecture fees from AstraZeneca.
Dr. David J. Maron reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Judith S. Hochman is Study Chair for the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial for which, in addition to support by a National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular, Medtronic, Inc, St Jude Medical Inc, Volcano Corporation, Arbor Pharmaceuticals LLC, AstraZeneca, Merck Sharp and Dohme Corp, Omron Healthcare Inc, and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca.
Figures






Comment in
-
Letter by Correia and Viana Regarding Article, "Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons".Circulation. 2021 Jul 13;144(2):e12-e13. doi: 10.1161/CIRCULATIONAHA.121.054697. Epub 2021 Jul 12. Circulation. 2021. PMID: 34251891 No abstract available.
-
Response by Chaitman et al to Letter Regarding Article, "Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons".Circulation. 2021 Jul 13;144(2):e14-e15. doi: 10.1161/CIRCULATIONAHA.121.055296. Epub 2021 Jul 12. Circulation. 2021. PMID: 34251894 No abstract available.
References
-
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction, Authors/Task Force Members Chairpersons, Thygesen K, Alpert JS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. - PubMed
-
- Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E and Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62:1563–70. - PMC - PubMed

