Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19
- PMID: 33267662
- PMCID: PMC7837697
- DOI: 10.1161/ATVBAHA.120.315267
Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19
Erratum in
-
Correction to: Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19.Arterioscler Thromb Vasc Biol. 2021 Jun;41(6):e384. doi: 10.1161/ATV.0000000000000144. Epub 2021 May 26. Arterioscler Thromb Vasc Biol. 2021. PMID: 34038168 No abstract available.
Abstract
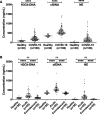
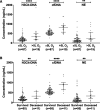
Objective: The full spectrum of coronavirus disease 2019 (COVID-19) infection ranges from asymptomatic to acute respiratory distress syndrome, characterized by hyperinflammation and thrombotic microangiopathy. The pathogenic mechanisms are poorly understood, but emerging evidence suggest that excessive neutrophil extracellular trap (NET) formation plays a key role in COVID-19 disease progression. Here, we evaluate if circulating markers of NETs are associated with COVID-19 disease severity and clinical outcome, as well as to markers of inflammation and in vivo coagulation and fibrinolysis. Approach and Results: One hundred six patients with COVID-19 with moderate to severe disease were enrolled shortly after hospital admission and followed for 4 months. Acute and convalescent plasma samples as well as plasma samples from 30 healthy individuals were assessed for markers of NET formation: citrullinated histone H3, cell-free DNA, NE (neutrophil elastase). We found that all plasma levels of NET markers were elevated in patients with COVID-19 relative to healthy controls, that they were associated with respiratory support requirement and short-term mortality, and declined to those found in healthy individuals 4 months post-infection. The levels of the NET markers also correlated with white blood cells, neutrophils, inflammatory cytokines, and C-reactive protein, as well as to markers of in vivo coagulation, fibrinolysis, and endothelial damage.
Conclusions: Our findings suggest a role of NETs in COVID-19 disease progression, implicating their contribution to an immunothrombotic state. Further, we observed an association between circulating markers of NET formation and clinical outcome, demonstrating a potential role of NET markers in clinical decision-making, as well as for NETs as targets for novel therapeutic interventions in COVID-19.
Keywords: Sars-Cov2; cytokines; extracellular traps; fibrinolysis; histone; immunothrombosis; thrombosis.
Conflict of interest statement
None.
Figures
Comment in
-
Neutrophil Extracellular Traps as Prognostic Markers in COVID-19: A Welcome Piece to the Puzzle.Arterioscler Thromb Vasc Biol. 2021 Feb;41(2):995-998. doi: 10.1161/ATVBAHA.120.315633. Epub 2021 Jan 27. Arterioscler Thromb Vasc Biol. 2021. PMID: 33955780 Free PMC article. No abstract available.
Similar articles
-
Neutrophil extracellular traps and macrophage activation contibute to thrombosis and post-covid syndrome in SARS-CoV-2 infection.Front Immunol. 2025 Feb 24;16:1507167. doi: 10.3389/fimmu.2025.1507167. eCollection 2025. Front Immunol. 2025. PMID: 40066452 Free PMC article.
-
Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.Blood. 2020 Sep 3;136(10):1169-1179. doi: 10.1182/blood.2020007008. Blood. 2020. PMID: 32597954 Free PMC article.
-
Prognostic value of circulating markers of neutrophil activation, neutrophil extracellular traps, coagulation and fibrinolysis in patients with terminal cancer.Sci Rep. 2021 Mar 3;11(1):5074. doi: 10.1038/s41598-021-84476-3. Sci Rep. 2021. PMID: 33658563 Free PMC article.
-
Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality.Int Immunopharmacol. 2022 Mar;104:108516. doi: 10.1016/j.intimp.2021.108516. Epub 2022 Jan 6. Int Immunopharmacol. 2022. PMID: 35032828 Free PMC article. Review.
-
Immunothrombosis in COVID-19: Implications of Neutrophil Extracellular Traps.Biomolecules. 2021 May 6;11(5):694. doi: 10.3390/biom11050694. Biomolecules. 2021. PMID: 34066385 Free PMC article. Review.
Cited by
-
Post-COVID-19 Syndrome in Outpatients and Its Association with Viral Load.Int J Environ Res Public Health. 2022 Nov 17;19(22):15145. doi: 10.3390/ijerph192215145. Int J Environ Res Public Health. 2022. PMID: 36429864 Free PMC article.
-
Do Circulating Histones Represent the Missing Link among COVID-19 Infection and Multiorgan Injuries, Microvascular Coagulopathy and Systemic Hyperinflammation?J Clin Med. 2022 Mar 24;11(7):1800. doi: 10.3390/jcm11071800. J Clin Med. 2022. PMID: 35407410 Free PMC article.
-
Biomarkers of cell damage, neutrophil and macrophage activation associated with in-hospital mortality in geriatric COVID-19 patients.Immun Ageing. 2022 Dec 15;19(1):65. doi: 10.1186/s12979-022-00315-7. Immun Ageing. 2022. PMID: 36522763 Free PMC article.
-
Casting a wide NET: an update on uncontrolled NETosis in response to COVID-19 infection.Clin Sci (Lond). 2022 Jul 15;136(13):1047-1052. doi: 10.1042/CS20220039. Clin Sci (Lond). 2022. PMID: 35791847 Free PMC article.
-
Self-DNA driven inflammation in COVID-19 and after mRNA-based vaccination: lessons for non-COVID-19 pathologies.Front Immunol. 2024 Feb 19;14:1259879. doi: 10.3389/fimmu.2023.1259879. eCollection 2023. Front Immunol. 2024. PMID: 38439942 Free PMC article.
References
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

