NADPH Oxidases Are Required for Full Platelet Activation In Vitro and Thrombosis In Vivo but Dispensable for Plasma Coagulation and Hemostasis
- PMID: 33267663
- PMCID: PMC7837688
- DOI: 10.1161/ATVBAHA.120.315565
NADPH Oxidases Are Required for Full Platelet Activation In Vitro and Thrombosis In Vivo but Dispensable for Plasma Coagulation and Hemostasis
Abstract
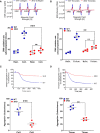
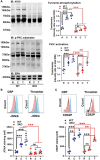
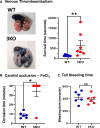
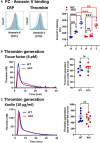
Objective: Using 3KO (triple NOX [NADPH oxidase] knockout) mice (ie, NOX1-/-/NOX2-/-/NOX4-/-), we aimed to clarify the role of this family of enzymes in the regulation of platelets in vitro and hemostasis in vivo. Approach and Results: 3KO mice displayed significantly reduced platelet superoxide radical generation, which was associated with impaired platelet aggregation, adhesion, and thrombus formation in response to the key agonists collagen and thrombin. A comparison with single-gene knockouts suggested that the phenotype of 3KO platelets is the combination of the effects of the genetic deletion of NOX1 and NOX2, while NOX4 does not show any significant function in platelet regulation. 3KO platelets displayed significantly higher levels of cGMP-a negative platelet regulator that activates PKG (protein kinase G). The inhibition of PKG substantially but only partially rescued the defective phenotype of 3KO platelets, which are responsive to both collagen and thrombin in the presence of the PKG inhibitors KT5823 or Rp-8-pCPT-cGMPs, but not in the presence of the NOS (NO synthase) inhibitor L-NG-monomethyl arginine. In vivo, triple NOX deficiency protected against ferric chloride-driven carotid artery thrombosis and experimental pulmonary embolism, while hemostasis tested in a tail-tip transection assay was not affected. Procoagulatory activity of platelets (ie, phosphatidylserine surface exposure) and the coagulation cascade in platelet-free plasma were normal.
Conclusions: This study indicates that inhibiting NOXs has strong antithrombotic effects partially caused by increased intracellular cGMP but spares hemostasis. NOXs are, therefore, pharmacotherapeutic targets to develop new antithrombotic drugs without bleeding side effects.
Keywords: NADPH oxidases; blood platelets; chlorides; oxidation-reduction; thrombosis.
Conflict of interest statement
None.
Figures


Similar articles
-
Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis.Arterioscler Thromb Vasc Biol. 2016 May;36(5):846-54. doi: 10.1161/ATVBAHA.116.307308. Epub 2016 Mar 17. Arterioscler Thromb Vasc Biol. 2016. PMID: 26988594 Free PMC article.
-
Class III PI3K Positively Regulates Platelet Activation and Thrombosis via PI(3)P-Directed Function of NADPH Oxidase.Arterioscler Thromb Vasc Biol. 2017 Nov;37(11):2075-2086. doi: 10.1161/ATVBAHA.117.309751. Epub 2017 Sep 7. Arterioscler Thromb Vasc Biol. 2017. PMID: 28882875
-
NADPH oxidase 1 is a novel pharmacological target for the development of an antiplatelet drug without bleeding side effects.FASEB J. 2020 Oct;34(10):13959-13977. doi: 10.1096/fj.202001086RRR. Epub 2020 Aug 26. FASEB J. 2020. PMID: 32851720
-
NADPH Oxidase-2 and Atherothrombosis: Insight From Chronic Granulomatous Disease.Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):218-225. doi: 10.1161/ATVBAHA.116.308351. Epub 2016 Dec 8. Arterioscler Thromb Vasc Biol. 2017. PMID: 27932349 Review.
-
NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis.Trends Cardiovasc Med. 2018 Oct;28(7):429-434. doi: 10.1016/j.tcm.2018.03.001. Epub 2018 Mar 26. Trends Cardiovasc Med. 2018. PMID: 29661712 Review.
Cited by
-
Role of Oxidative Stress in the Pathogenesis of Atherothrombotic Diseases.Antioxidants (Basel). 2022 Jul 20;11(7):1408. doi: 10.3390/antiox11071408. Antioxidants (Basel). 2022. PMID: 35883899 Free PMC article. Review.
-
Evaluation of the Potential Beneficial Effects of Ferula communis L. Extract Supplementation in Postmenopausal Discomfort.Nutrients. 2024 Aug 11;16(16):2651. doi: 10.3390/nu16162651. Nutrients. 2024. PMID: 39203788 Free PMC article. Clinical Trial.
-
The Efficacy of the Systemic Immune-Inflammation Index and Prognosis Nutritional Index for the Diagnosis of Venous Thromboembolism in Gastrointestinal Cancers.J Inflamm Res. 2022 Aug 15;15:4649-4661. doi: 10.2147/JIR.S376601. eCollection 2022. J Inflamm Res. 2022. PMID: 35996687 Free PMC article.
-
Protein disulfide isomerase-A1 regulates intraplatelet reactive oxygen species-thromboxane A2 -dependent pathway in human platelets.J Thromb Haemost. 2022 Jan;20(1):157-169. doi: 10.1111/jth.15539. Epub 2021 Oct 14. J Thromb Haemost. 2022. PMID: 34592041 Free PMC article.
-
Understanding the Role of Oxidative Stress in Platelet Alterations and Thrombosis Risk among Frail Older Adults.Biomedicines. 2024 Sep 3;12(9):2004. doi: 10.3390/biomedicines12092004. Biomedicines. 2024. PMID: 39335518 Free PMC article. Review.
References
-
- Begonja AJ, Teichmann L, Geiger J, Gambaryan S, Walter U. Platelet regulation by NO/cGMP signaling and NAD(P)H oxidase-generated ROS. Blood Cells Mol Dis. 2006;36:166–170. doi: 10.1016/j.bcmd.2005.12.028 - PubMed
-
- Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047 - PubMed
-
- Chlopicki S, Olszanecki R, Janiszewski M, Laurindo FR, Panz T, Miedzobrodzki J. Functional role of NADPH oxidase in activation of platelets. Antioxid Redox Signal. 2004;6:691–698. doi: 10.1089/1523086041361640 - PubMed
-
- Carnevale R, Loffredo L, Sanguigni V, Plebani A, Rossi P, Pignata C, Martire B, Finocchi A, Pietrogrande MC, Azzari C, et al. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: lesson from chronic granulomatous disease. J Am Heart Assoc. 2014;3:e000920 doi: 10.1161/JAHA.114.000920 - PMC - PubMed
-
- Vara D, Cifuentes-Pagano E, Pagano PJ, Pula G. A novel combinatorial technique for simultaneous quantification of oxygen radicals and aggregation reveals unexpected redox patterns in the activation of platelets by different physiopathological stimuli. Haematologica. 2019;104:1879–1891. doi: 10.3324/haematol.2018.208819 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

