LAD1 expression is associated with the metastatic potential of colorectal cancer cells
- PMID: 33267790
- PMCID: PMC7709356
- DOI: 10.1186/s12885-020-07660-0
LAD1 expression is associated with the metastatic potential of colorectal cancer cells
Abstract
Background: Anchoring filament protein ladinin-1 (LAD1) was related to the aggressive progression of breast, lung, laryngeal and thyroid cancers. However, the association of LAD1 with colorectal cancer remained unknown. Here, to determine the relationship of LAD1 with colorectal cancer progression, we explored the effect of LAD1 loss on the malignant features of colorectal cancer cells.
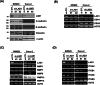
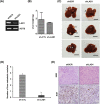
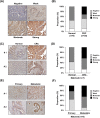
Methods: We constructed LAD1-depleted cell lines and examined the effect of LAD1 deficiency on the phenotypic and molecular features of colorectal cancer cells in vitro. The function of LAD1 in metastasis in vivo was examined by establishing a spleen-to-liver metastasis mouse model. LAD1 protein expression in colorectal cancer patient specimens was assessed by immunohistochemistry of tumor microarrays.
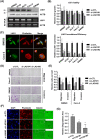
Results: We found that LAD1 was abundant in most colorectal cancer cells. In addition, high expression of LAD1 significantly correlated with poor patient outcome. LAD1 depletion inhibited the migration and invasion of two different colorectal cancer cell lines, SW620 and Caco-2, without affecting their proliferation. In addition, LAD1 loss led to defects in liver metastasis of SW620 cells in the mouse model. Immunohistochemistry of colorectal cancer tissues revealed LAD1 enrichment in metastatic tissues compared to that in primary tumor and normal tissues.
Conclusion: These results suggest that LAD1 expression is associated with the metastatic progression of colorectal cancer by promoting the migration and invasion of cancer cells.
Keywords: Colorectal cancer; Invasion; Ladinin-1; Metastasis; Migration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Teixeira JC, de Filippo C, Weihmann A, Meneu JR, Racimo F, Dannemann M, Nickel B, Fischer A, Halbwax M, Andre C, et al. Long-term balancing selection in LAD1 maintains a missense trans-species polymorphism in humans, chimpanzees, and bonobos. Mol Biol Evol. 2015;32(5):1186–1196. doi: 10.1093/molbev/msv007. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

