Bladder cancer-derived interleukin-1 converts the vascular endothelium into a pro-inflammatory and pro-coagulatory surface
- PMID: 33267794
- PMCID: PMC7709388
- DOI: 10.1186/s12885-020-07548-z
Bladder cancer-derived interleukin-1 converts the vascular endothelium into a pro-inflammatory and pro-coagulatory surface
Abstract
Background: Bladder cancer cells orchestrate tumour progression by pro-inflammatory cytokines. Cytokines modulate the local tumour microenvironment and increase the susceptibility of tumour distant tissues for metastasis. Here, we investigated the impact of human bladder cancer cell derived factors on the ability to modulate and activate human vascular endothelial cells.
Methods: The pro-inflammatory and pro-coagulatory potential of four different bladder cancer cell lines was accessed by qRT-PCR arrays and ELISA. Modulation and activation of endothelial cells was studied in microfluidic devices. Clinical relevance of our findings was confirmed by immune histology in tissue samples of bladder cancer patients and public transcriptome data.
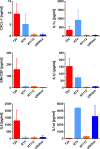
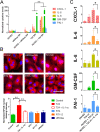
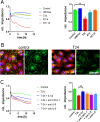
Results: The unbalanced ratio between interleukin (IL)-1 and IL-1 receptor antagonist (IL-1ra) in the secretome of bladder cancer cells converted the quiescent vascular endothelium into a pro-adhesive, pro-inflammatory, and pro-coagulatory surface. Microfluidic experiments showed that tumour cell induced endothelial cell activation promoted leukocyte recruitment and platelet adhesion. Human bladder cancer tissue analysis confirmed that loss of IL-1ra and elevated IL-1 expression was associated with enhanced cancer progression.
Conclusions: Our data indicate that IL-1 and IL-1ra were dysregulated in bladder cancer and could facilitate tumour dissemination through endothelial cell activation. Targeting the IL-1/IL-1ra axis might attenuate tumour-mediated inflammation and metastasis formation.
Keywords: Coagulation; Endothelial cells; Inflammation; Tumour microenvironment; von Willebrand factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. - PubMed
-
- Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol. 2017; nrurol. 2017.2179. - PubMed
-
- Young A, Chapman O, Connor C, Poole C, Rose P, Kakkar AK. Thrombosis and cancer. Nat Rev Clin Oncol. 2012;9(8):437–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

