Novel therapeutics in myeloproliferative neoplasms
- PMID: 33267911
- PMCID: PMC7709419
- DOI: 10.1186/s13045-020-00995-y
Novel therapeutics in myeloproliferative neoplasms
Abstract
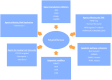
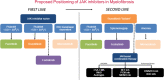
Hyperactive signaling of the Janus-Associated Kinase/Signal Transducers and Activators of Transcription (JAK/STAT) pathway is central to the pathogenesis of Philadelphia-chromosome-negative myeloproliferative neoplasms (MPN), i.e., polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) which are characterized by inherent biological and clinical heterogeneity. Patients with MPNs suffer from substantial symptom burden and curtailed longevity due to thrombohemorrhagic complications or progression to myelofibrosis or acute myeloid leukemia. Therefore, the management strategies focus on thrombosis risk mitigation in PV/ET, alleviation of symptom burden and improvement in cytopenias and red blood cell transfusion requirements, and disease course alteration in PMF. The United States Food and Drug Administration's (USFDA) approval of two JAK inhibitors (ruxolitinib, fedratinib) has transformed the therapeutic landscape of MPNs in assuaging the need for frequent therapeutic phlebotomy (PV) and reduction in spleen and symptom burden (PV and PMF). Despite improving biological understanding of these complex clonal hematopoietic stem/progenitor cell neoplasms, none of the currently available therapies appear to modify the proclivity of the disease per se, thereby remaining an urgent unmet clinical need and an ongoing area of intense clinical investigation. This review will highlight the evolving targeted therapeutic agents that are in early- and late-stage MPN clinical development.
Keywords: CALR; CALR vaccine; ET; Fedratinib; Imetelstat; JAK-STAT; MF; PV; Pacritinib; Ruxolitinib.
Conflict of interest statement
John Mascarenhas has received research funding paid to the institution from Incyte, Roche, CTI Biopharma, Novartis, Promedior, Merck, Janssen, Arog, Merus, and Pharma Essentia, Kartos, Forbius; consulting fees from Geron, Constellation, Prelude, Galecto, Promedior, and Celgene/BMS, Kartos. Sangeetha Venugopal has nothing to disclose.
Figures
Similar articles
-
The BCR-ABL1-negative myeloproliferative neoplasms: a review of JAK inhibitors in the therapeutic armamentarium.Expert Opin Pharmacother. 2017 Dec;18(18):1929-1938. doi: 10.1080/14656566.2017.1404574. Epub 2017 Nov 26. Expert Opin Pharmacother. 2017. PMID: 29134817 Review.
-
Individualizing Care for Patients With Myeloproliferative Neoplasms: Integrating Genetics, Evolving Therapies, and Patient-Specific Disease Burden.Am Soc Clin Oncol Educ Book. 2016;35:e324-35. doi: 10.1200/EDBK_159322. Am Soc Clin Oncol Educ Book. 2016. PMID: 27249739 Review.
-
JAK2 inhibitors for myeloproliferative neoplasms: what is next?Blood. 2017 Jul 13;130(2):115-125. doi: 10.1182/blood-2017-04-742288. Epub 2017 May 12. Blood. 2017. PMID: 28500170 Free PMC article. Review.
-
Investigational histone deacetylase inhibitors (HDACi) in myeloproliferative neoplasms.Expert Opin Investig Drugs. 2016 Dec;25(12):1393-1403. doi: 10.1080/13543784.2016.1250882. Epub 2016 Oct 31. Expert Opin Investig Drugs. 2016. PMID: 27756180 Free PMC article. Review.
-
New Strategies in Myeloproliferative Neoplasms: The Evolving Genetic and Therapeutic Landscape.Clin Cancer Res. 2016 Mar 1;22(5):1037-47. doi: 10.1158/1078-0432.CCR-15-0905. Clin Cancer Res. 2016. PMID: 26933174 Free PMC article. Review.
Cited by
-
JAK2 inhibitors for the treatment of Philadelphia-negative myeloproliferative neoplasms: current status and future directions.Mol Divers. 2024 Oct;28(5):3445-3456. doi: 10.1007/s11030-023-10742-3. Epub 2023 Nov 25. Mol Divers. 2024. PMID: 38006563 Review.
-
Allogeneic stem cell transplantation may overcome the adverse impact of myelofibrosis on the prognosis of myelodysplastic syndrome.Exp Hematol Oncol. 2021 Aug 14;10(1):44. doi: 10.1186/s40164-021-00238-x. Exp Hematol Oncol. 2021. PMID: 34391477 Free PMC article.
-
Shaping the Future of Myeloproliferative Neoplasm Therapy: Immune-Based Strategies and Targeted Innovations.Cancers (Basel). 2024 Dec 8;16(23):4113. doi: 10.3390/cancers16234113. Cancers (Basel). 2024. PMID: 39682299 Free PMC article. Review.
-
Fusion transcripts FYN-TRAF3IP2 and KHDRBS1-LCK hijack T cell receptor signaling in peripheral T-cell lymphoma, not otherwise specified.Nat Commun. 2021 Jun 17;12(1):3705. doi: 10.1038/s41467-021-24037-4. Nat Commun. 2021. PMID: 34140493 Free PMC article.
-
SOHO State of the Art Updates and Next Questions: Novel Therapeutic Strategies in Development for Myelofibrosis.Clin Lymphoma Myeloma Leuk. 2023 Apr;23(4):219-231. doi: 10.1016/j.clml.2022.12.014. Epub 2023 Jan 6. Clin Lymphoma Myeloma Leuk. 2023. PMID: 36797153 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

