Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles
- PMID: 33268351
- PMCID: PMC7713199
- DOI: 10.1136/jitc-2020-001372
Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles
Abstract
Background: Mitochondrial Lon is a chaperone and DNA-binding protein that functions in protein quality control and stress response pathways. The level of Lon regulates mitochondrial DNA (mtDNA) metabolism and the production of mitochondrial reactive oxygen species (ROS). However, there is little information in detail on how mitochondrial Lon regulates ROS-dependent cancer immunoescape through mtDNA metabolism in the tumor microenvironment (TME).
Methods: We explored the understanding of the intricate interplay between mitochondria and the innate immune response in the inflammatory TME.
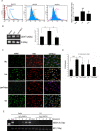
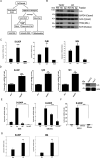
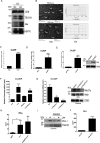
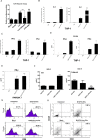
Results: We found that oxidized mtDNA is released into the cytosol when Lon is overexpressed and then it induces interferon (IFN) signaling via cGAS-STING-TBK1, which upregulates PD-L1 and IDO-1 expression to inhibit T-cell activation. Unexpectedly, upregulation of Lon also induces the secretion of extracellular vehicles (EVs), which carry mtDNA and PD-L1. Lon-induced EVs further induce the production of IFN and IL-6 from macrophages, which attenuates T-cell immunity in the TME.
Conclusions: The levels of mtDNA and PD-L1 in EVs in patients with oral cancer function as a potential diagnostic biomarker for anti-PD-L1 immunotherapy. Our studies provide an insight into the immunosuppression on mitochondrial stress and suggest a therapeutic synergy between anti-inflammation therapy and immunotherapy in cancer.
Keywords: inflammation; interferon inducers; tumor biomarkers; tumor escape; tumor microenvironment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
