Leapfrogging laboratories: the promise and pitfalls of high-tech solutions for antimicrobial resistance surveillance in low-income settings
- PMID: 33268385
- PMCID: PMC7712442
- DOI: 10.1136/bmjgh-2020-003622
Leapfrogging laboratories: the promise and pitfalls of high-tech solutions for antimicrobial resistance surveillance in low-income settings
Abstract
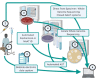
The scope and trajectory of today's escalating antimicrobial resistance (AMR) crisis is inadequately captured by existing surveillance systems, particularly those of lower income settings. AMR surveillance systems typically collate data from routine culture and susceptibility testing performed in diagnostic bacteriology laboratories to support healthcare. Limited access to high quality culture and susceptibility testing results in the dearth of AMR surveillance data, typical of many parts of the world where the infectious disease burden and antimicrobial need are high. Culture and susceptibility testing by traditional techniques is also slow, which limits its value in infection management. Here, we outline hurdles to effective resistance surveillance in many low-income settings and encourage an open attitude towards new and evolving technologies that, if adopted, could close resistance surveillance gaps. Emerging advancements in point-of-care testing, laboratory detection of resistance through or without culture, and in data handling, have the potential to generate resistance data from previously unrepresented locales while simultaneously supporting healthcare. Among them are microfluidic, nucleic acid amplification technology and next-generation sequencing approaches. Other low tech or as yet unidentified innovations could also rapidly accelerate AMR surveillance. Parallel advances in data handling further promise to significantly improve AMR surveillance, and new frameworks that can capture, collate and use alternate data formats may need to be developed. We outline the promise and limitations of such technologies, their potential to leapfrog surveillance over currently available, conventional technologies in use today and early steps that health systems could take towards preparing to adopt them.
Keywords: disease; disorder; epidemiology; health systems; medical microbiology; or injury; other diagnostic or tool; other infection.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JP reports personal fees from NextGen Diagnostic, outside the submitted work; other authors have no competing interests to declare.
Figures
References
-
- WHO Antimicrobial resistance: global report on surveillance 2014 2014:257.
-
- Okeke IN. Divining without seeds : the case for strengthening laboratory medicine in Africa. Ithaca: ILR/ Cornell University Press, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
