Starvation Responses Throughout the Caenorhabditiselegans Life Cycle
- PMID: 33268389
- PMCID: PMC7768255
- DOI: 10.1534/genetics.120.303565
Starvation Responses Throughout the Caenorhabditiselegans Life Cycle
Abstract
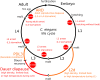
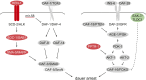
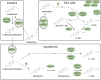
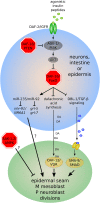
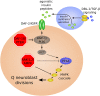
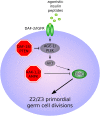
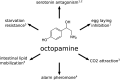
Caenorhabditis elegans survives on ephemeral food sources in the wild, and the species has a variety of adaptive responses to starvation. These features of its life history make the worm a powerful model for studying developmental, behavioral, and metabolic starvation responses. Starvation resistance is fundamental to life in the wild, and it is relevant to aging and common diseases such as cancer and diabetes. Worms respond to acute starvation at different times in the life cycle by arresting development and altering gene expression and metabolism. They also anticipate starvation during early larval development, engaging an alternative developmental program resulting in dauer diapause. By arresting development, these responses postpone growth and reproduction until feeding resumes. A common set of signaling pathways mediates systemic regulation of development in each context but with important distinctions. Several aspects of behavior, including feeding, foraging, taxis, egg laying, sleep, and associative learning, are also affected by starvation. A variety of conserved signaling, gene regulatory, and metabolic mechanisms support adaptation to starvation. Early life starvation can have persistent effects on adults and their descendants. With its short generation time, C. elegans is an ideal model for studying maternal provisioning, transgenerational epigenetic inheritance, and developmental origins of adult health and disease in humans. This review provides a comprehensive overview of starvation responses throughout the C. elegans life cycle.
Keywords: L1 arrest; WormBook; dauer; quiescence; starvation.
Copyright © 2020 by the Genetics Society of America.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

