Immune response profiling of patients with spondyloarthritis reveals signalling networks mediating TNF-blocker function in vivo
- PMID: 33268443
- PMCID: PMC7958106
- DOI: 10.1136/annrheumdis-2020-218304
Immune response profiling of patients with spondyloarthritis reveals signalling networks mediating TNF-blocker function in vivo
Abstract
Objectives: Antitumour necrosis factor (TNF) therapy has revolutionised treatment of several chronic inflammatory diseases, including spondyloarthritis (SpA). However, TNF inhibitors (TNFi) are not effective in all patients and the biological basis for treatment failure remains unknown. We have analysed induced immune responses to define the mechanism of action of TNF blockers in SpA and to identify immunological correlates of responsiveness to TNFi.
Methods: Immune responses to microbial and pathway-specific stimuli were analysed in peripheral blood samples from 80 patients with axial SpA before and after TNFi treatment, using highly standardised whole-blood stimulation assays. Cytokines and chemokines were measured in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory, and gene expression was monitored using nCounter assays.
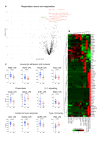
Results: Anti-TNF therapy induced profound changes in patients' innate immune responses. TNFi action was selective, and had only minor effects on Th1/Th17 immunity. Modular transcriptional repertoire analysis identified prostaglandin E2 synthesis and signalling, leucocyte recirculation, macrophage polarisation, dectin and interleukin (IL)-1 signalling, as well as the nuclear factor kappa B (NF-kB) transcription factor family as key pathways targeted by TNF blockers in vivo. Analysis of induced immune responses before treatment initiation revealed that expression of molecules associated with leucocyte adhesion and invasion, chemotaxis and IL-1 signalling are correlated with therapeutic responses to anti-TNF.
Conclusions: We show that TNFi target multiple immune cell pathways that cooperate to resolve inflammation. We propose that immune response profiling provides new insight into the biology of TNF-blocker action in patients and can identify signalling pathways associated with therapeutic responses to biological therapies.
Keywords: ankylosing; biological therapy; immune system diseases; spondylitis; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

