Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2
- PMID: 33268510
- PMCID: PMC8056991
- DOI: 10.1126/scitranslmed.abb5413
Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2
Abstract
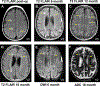
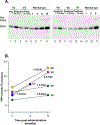
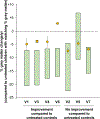
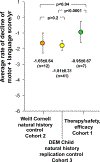
Late infantile Batten disease (CLN2 disease) is an autosomal recessive, neurodegenerative lysosomal storage disease caused by mutations in the CLN2 gene encoding tripeptidyl peptidase 1 (TPP1). We tested intraparenchymal delivery of AAVrh.10hCLN2, a nonhuman serotype rh.10 adeno-associated virus vector encoding human CLN2, in a nonrandomized trial consisting of two arms assessed over 18 months: AAVrh.10hCLN2-treated cohort of 8 children with mild to moderate disease and an untreated, Weill Cornell natural history cohort consisting of 12 children. The treated cohort was also compared to an untreated European natural history cohort of CLN2 disease. The vector was administered through six burr holes directly to 12 sites in the brain without immunosuppression. In an additional safety assessment under a separate protocol, five children with severe CLN2 disease were treated with AAVrh.10hCLN2. The therapy was associated with a variety of expected adverse events, none causing long-term disability. Induction of systemic anti-AAVrh.10 immunity was mild. After therapy, the treated cohort had a 1.3- to 2.6-fold increase in cerebral spinal fluid TPP1. There was a slower loss of gray matter volume in four of seven children by MRI and a 42.4 and 47.5% reduction in the rate of decline of motor and language function, compared to Weill Cornell natural history cohort (P < 0.04) and European natural history cohort (P < 0.0001), respectively. Intraparenchymal brain administration of AAVrh.10hCLN2 slowed the progression of disease in children with CLN2 disease. However, improvements in vector design and delivery strategies will be necessary to halt disease progression using gene therapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

References
-
- Williams RE, Gottlob I, Lake BD, Goebel HH, Wheeler W, Wheeler RB, in The Neuronal Ceroid Lipofuscinoses (Batten Disease), Goebel HH, Mole SE, Lake BD, Eds. (IOS Press, Amsterdam, 1999), pp. 37–54.
-
- Worgall S, Kekatpure MV, Heier L, Ballon D, Dyke JP, Shungu D, Mao X, Kosofsky B, Kaplitt MG, Souweidane MM, Sondhi D, Hackett NR, Hollmann C, Crystal RG, Neurological deterioration in late infantile neuronal ceroid lipofuscinosis. Neurology 69, 521–535 (2007); published online EpubAug 7 (10.1212/01.wnl.0000267885.47092.40). - DOI - PubMed
-
- Nickel M, Simonati A, Jacoby D, Lezius S, Kilian D, Van de Graaf B, Pagovich OE, Kosofsky B, Yohay K, Downs M, Slasor P, Ajayi T, Crystal RG, Kohlschutter A, Sondhi D, Schulz A, Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: an observational cohort study. Lancet Child Adolesc Health 2, 582–590 (2018); published online EpubAug (10.1016/S2352-4642(18)30179-2). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

