Chronic embedded cortico-thalamic closed-loop deep brain stimulation for the treatment of essential tremor
- PMID: 33268512
- PMCID: PMC8182660
- DOI: 10.1126/scitranslmed.aay7680
Chronic embedded cortico-thalamic closed-loop deep brain stimulation for the treatment of essential tremor
Abstract
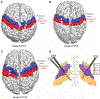
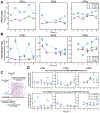
Deep brain stimulation (DBS) is an approved therapy for the treatment of medically refractory and severe movement disorders. However, most existing neurostimulators can only apply continuous stimulation [open-loop DBS (OL-DBS)], ignoring patient behavior and environmental factors, which consequently leads to an inefficient therapy, thus limiting the therapeutic window. Here, we established the feasibility of a self-adjusting therapeutic DBS [closed-loop DBS (CL-DBS)], fully embedded in a chronic investigational neurostimulator (Activa PC + S), for three patients affected by essential tremor (ET) enrolled in a longitudinal (6 months) within-subject crossover protocol (DBS OFF, OL-DBS, and CL-DBS). Most patients with ET experience involuntary limb tremor during goal-directed movements, but not during rest. Hence, the proposed CL-DBS paradigm explored the efficacy of modulating the stimulation amplitude based on patient-specific motor behavior, suppressing the pathological tremor on-demand based on a cortical electrode detecting upper limb motor activity. Here, we demonstrated how the proposed stimulation paradigm was able to achieve clinical efficacy and tremor suppression comparable with OL-DBS in a range of movements (cup reaching, proximal and distal posture, water pouring, and writing) while having a consistent reduction in energy delivery. The proposed paradigm is an important step toward a behaviorally modulated fully embedded DBS system, capable of delivering stimulation only when needed, and potentially mitigating pitfalls of OL-DBS, such as DBS-induced side effects and premature device replacement.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




References
-
- Mücke D, Becker J, Barbe MT, Meister I, Liebhart L, Roettger TB, Dembek T, Timmermann L, Grice M, The effect of deep brain stimulation on the speech motor system. J. Speech Lang. Hear. Res. 57, 1206–1218 (2014). - PubMed
-
- Zesiewicz TA, Shaw JD, Allison KG, Staffetti JS, Okun MS, Sullivan KL, Update on treatment of essential tremor. Curr. Treat. Options Neurol. 15, 410–423 (2013). - PubMed
-
- Mücke D, Hermes A, Roettger TB, Becker J, Niemann H, Dembek TA, Timmermann L, Visser-Vandewalle V, Fink GR, Grice M, Barbe MT, Gonzalez-Alegre P, The effects of thalamic deep brain stimulation on speech dynamics in patients with essential tremor: An articulographic study. PLOS ONE 13, e0191359 (2018). - PMC - PubMed
-
- Mitchell KT, Larson P, Starr PA, Okun MS, Wharen RE Jr., Uitti RJ, Guthrie BL, Peichel D, Pahwa R, Walker HC, Foote K, Marshall FJ, Jankovic J, Simpson R, Phibbs F, Neimat JS, Stewart RM, Dashtipour K, Ostrem JL, Benefits and risks of unilateral and bilateral ventral intermediate nucleus deep brain stimulation for axial essential tremor symptoms. Parkinsonism Relat. Disord. 60, 126–132 (2019). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

