Regulation of VP30-Dependent Transcription by RNA Sequence and Structure in the Genomic Ebola Virus Promoter
- PMID: 33268520
- PMCID: PMC8092829
- DOI: 10.1128/JVI.02215-20
Regulation of VP30-Dependent Transcription by RNA Sequence and Structure in the Genomic Ebola Virus Promoter
Erratum in
-
Erratum for Bach et al., "Regulation of VP30-Dependent Transcription by RNA Sequence and Structure in the Genomic Ebola Virus Promoter".J Virol. 2023 Oct 31;97(10):e0125623. doi: 10.1128/jvi.01256-23. Epub 2023 Oct 3. J Virol. 2023. PMID: 37787531 Free PMC article. No abstract available.
Abstract
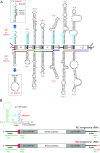
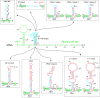
Viral transcription and replication of Ebola virus (EBOV) is balanced by transcription factor VP30, an RNA binding protein. An RNA hairpin at the transcription start site (TSS) of the first gene (NP hairpin) in the 3'-leader promoter is thought to mediate the VP30 dependency of transcription. Here, we investigated the constraints of VP30 dependency using a series of monocistronic minigenomes with sequence, structure and length deviations from the native NP hairpin. Hairpin stabilizations decreased while destabilizations increased transcription in the absence of VP30, but in all cases, transcription activity was higher in the presence versus absence of VP30. This also pertains to a mutant that is unable to form any RNA secondary structure at the TSS, demonstrating that the activity of VP30 is not simply determined by the capacity to form a hairpin structure at the TSS. Introduction of continuous 3'-UN5 hexamer phasing between promoter elements PE1 and PE2 by a single point mutation in the NP hairpin boosted VP30-independent transcription. Moreover, this point mutation, but also hairpin stabilizations, impaired the relative increase of replication in the absence of VP30. Our results suggest that the native NP hairpin is optimized for tight regulation by VP30 while avoiding an extent of hairpin stability that impairs viral transcription, as well as for enabling the switch from transcription to replication when VP30 is not part of the polymerase complex.IMPORTANCE A detailed understanding is lacking how the Ebola virus (EBOV) protein VP30 regulates activity of the viral polymerase complex. Here, we studied how RNA sequence, length and structure at the transcription start site (TSS) in the 3'-leader promoter influence the impact of VP30 on viral polymerase activity. We found that hairpin stabilizations tighten the VP30 dependency of transcription but reduce transcription efficiency and attenuate the switch to replication in the absence of VP30. Upon hairpin destabilization, VP30-independent transcription - already weakly detectable at the native promoter - increases, but never reaches the same extent as in the presence of VP30. We conclude that the native hairpin structure involving the TSS (i) establishes an optimal balance between efficient transcription and tight regulation by VP30, (ii) is linked to hexamer phasing in the promoter, and (iii) favors the switch to replication when VP30 is absent.
Copyright © 2020 American Society for Microbiology.
Figures


Similar articles
-
RNA secondary structure at the transcription start site influences EBOV transcription initiation and replication in a length- and stability-dependent manner.RNA Biol. 2021 Apr;18(4):523-536. doi: 10.1080/15476286.2020.1818459. Epub 2020 Oct 22. RNA Biol. 2021. PMID: 32882148 Free PMC article.
-
Hexamer phasing governs transcription initiation in the 3'-leader of Ebola virus.RNA. 2020 Apr;26(4):439-453. doi: 10.1261/rna.073718.119. Epub 2020 Jan 10. RNA. 2020. PMID: 31924730 Free PMC article.
-
RNA Binding of Ebola Virus VP30 Is Essential for Activating Viral Transcription.J Virol. 2016 Jul 27;90(16):7481-7496. doi: 10.1128/JVI.00271-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27279615 Free PMC article.
-
RNA binding specificity of Ebola virus transcription factor VP30.RNA Biol. 2016 Sep;13(9):783-98. doi: 10.1080/15476286.2016.1194160. Epub 2016 Jun 17. RNA Biol. 2016. PMID: 27315567 Free PMC article.
-
Structural and Functional Aspects of Ebola Virus Proteins.Pathogens. 2021 Oct 15;10(10):1330. doi: 10.3390/pathogens10101330. Pathogens. 2021. PMID: 34684279 Free PMC article. Review.
Cited by
-
Identification and characterization of short leader and trailer RNAs synthesized by the Ebola virus RNA polymerase.PLoS Pathog. 2021 Oct 26;17(10):e1010002. doi: 10.1371/journal.ppat.1010002. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34699554 Free PMC article.
-
Marburg Virus VP30 Is Required for Transcription Initiation at the Glycoprotein Gene.mBio. 2022 Oct 26;13(5):e0224322. doi: 10.1128/mbio.02243-22. Epub 2022 Aug 23. mBio. 2022. PMID: 35997284 Free PMC article.
-
How does the polymerase of non-segmented negative strand RNA viruses commit to transcription or genome replication?J Virol. 2024 Aug 20;98(8):e0033224. doi: 10.1128/jvi.00332-24. Epub 2024 Jul 30. J Virol. 2024. PMID: 39078194 Free PMC article. Review.
-
Emerging roles of the Protein Phosphatase 1 (PP1) in the context of viral infections.Cell Commun Signal. 2024 Jan 24;22(1):65. doi: 10.1186/s12964-023-01468-8. Cell Commun Signal. 2024. PMID: 38267954 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

