Matrix Protein 2 Extracellular Domain-Specific Monoclonal Antibodies Are an Effective and Potentially Universal Treatment for Influenza A
- PMID: 33268521
- PMCID: PMC8092830
- DOI: 10.1128/JVI.01027-20
Matrix Protein 2 Extracellular Domain-Specific Monoclonal Antibodies Are an Effective and Potentially Universal Treatment for Influenza A
Erratum in
-
Correction for Bimler et al., "Matrix Protein 2 Extracellular Domain-Specific Monoclonal Antibodies Are an Effective and Potentially Universal Treatment for Influenza A".J Virol. 2022 Oct 12;96(19):e0135822. doi: 10.1128/jvi.01358-22. Epub 2022 Sep 27. J Virol. 2022. PMID: 36165629 Free PMC article. No abstract available.
Abstract
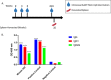
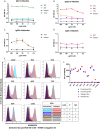
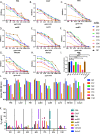
Influenza virus infection causes significant morbidity and mortality worldwide. Humans fail to make a universally protective memory immune response to influenza A. Hemagglutinin and Neuraminidase undergo antigenic drift and shift, resulting in new influenza A strains to which humans are naive. Seasonal vaccines are often ineffective and escape mutants have been reported to all treatments for influenza A. In the absence of a universal influenza A vaccine or treatment, influenza A will remain a significant threat to human health. The extracellular domain of the M2-ion channel (M2e) is an ideal antigenic target for a universal therapeutic agent, as it is highly conserved across influenza A serotypes, has a low mutation rate, and is essential for viral entry and replication. Previous M2e-specific monoclonal antibodies (M2e-MAbs) show protective potential against influenza A, however, they are either strain specific or have limited efficacy. We generated seven murine M2e-MAbs and utilized in vitro and in vivo assays to validate the specificity of our novel M2e-MAbs and to explore the universality of their protective potential. Our data shows our M2e-MAbs bind to M2e peptide, HEK cells expressing the M2 channel, as well as, influenza virions and MDCK-ATL cells infected with influenza viruses of multiple serotypes. Our antibodies significantly protect highly influenza A virus susceptible BALB/c mice from lethal challenge with H1N1 A/PR/8/34, pH1N1 A/CA/07/2009, H5N1 A/Vietnam/1203/2004, and H7N9 A/Anhui/1/2013 by improving survival rates and weight loss. Based on these results, at least four of our seven M2e-MAbs show strong potential as universal influenza A treatments.IMPORTANCE Despite a seasonal vaccine and multiple therapeutic treatments, Influenza A remains a significant threat to human health. The biggest obstacle is producing a vaccine or treatment for influenza A is their universality or efficacy against not only seasonal variances in the influenza virus, but also against all human, avian, and swine serotypes and, therefore, potential pandemic strains. M2e has huge potential as a target for a vaccine or treatment against influenza A. It is the most conserved external protein on the virus. Antibodies against M2e have made it to clinical trials, but not succeeded. Here, we describe novel M2e antibodies produced in mice that are not only protective at low doses, but that we extensively test to determine their universality and found to be cross protective against all strains tested. Additionally, our work begins to elucidate the critical role of isotype for an influenza A monoclonal antibody therapeutic.
Copyright © 2020 American Society for Microbiology.
Figures





Similar articles
-
AuNP-M2e + sCpG vaccination of juvenile mice generates lifelong protective immunity to influenza A virus infection.Immun Ageing. 2019 Sep 2;16:23. doi: 10.1186/s12979-019-0162-y. eCollection 2019. Immun Ageing. 2019. PMID: 31507643 Free PMC article.
-
An H5N1-based matrix protein 2 ectodomain tetrameric peptide vaccine provides cross-protection against lethal infection with H7N9 influenza virus.Emerg Microbes Infect. 2015 Apr;4(4):e22. doi: 10.1038/emi.2015.22. Epub 2015 Apr 8. Emerg Microbes Infect. 2015. PMID: 26038770 Free PMC article.
-
An H5N1 M2e-based multiple antigenic peptide vaccine confers heterosubtypic protection from lethal infection with pandemic 2009 H1N1 virus.Virol J. 2010 Jul 12;7:151. doi: 10.1186/1743-422X-7-151. Virol J. 2010. PMID: 20624292 Free PMC article.
-
M2e-based universal influenza A vaccine.Vaccine. 2009 Oct 23;27(45):6280-3. doi: 10.1016/j.vaccine.2009.07.007. Vaccine. 2009. PMID: 19840661 Review.
-
Mechanisms of Cross-protection by Influenza Virus M2-based Vaccines.Immune Netw. 2015 Oct;15(5):213-21. doi: 10.4110/in.2015.15.5.213. Epub 2015 Oct 26. Immune Netw. 2015. PMID: 26557805 Free PMC article. Review.
Cited by
-
Evolutionary and functional conservation of IRF7 in the Tibetan frog Nanorana parkeri.Mol Biol Rep. 2024 Jan 16;51(1):114. doi: 10.1007/s11033-023-09067-z. Mol Biol Rep. 2024. PMID: 38227268
-
Requirement of Fc-Fc Gamma Receptor Interaction for Antibody-Based Protection against Emerging Virus Infections.Viruses. 2021 May 31;13(6):1037. doi: 10.3390/v13061037. Viruses. 2021. PMID: 34072720 Free PMC article. Review.
-
Drosophila as a Model for Human Viral Neuroinfections.Cells. 2022 Aug 29;11(17):2685. doi: 10.3390/cells11172685. Cells. 2022. PMID: 36078091 Free PMC article. Review.
-
Enhancing neutralizing activity against influenza H1N1/PR8 by engineering a single-domain VL-M2 specific into a bivalent form.PLoS One. 2022 Aug 31;17(8):e0273934. doi: 10.1371/journal.pone.0273934. eCollection 2022. PLoS One. 2022. PMID: 36044435 Free PMC article.
-
Antiviral Approaches against Influenza Virus.Clin Microbiol Rev. 2023 Mar 23;36(1):e0004022. doi: 10.1128/cmr.00040-22. Epub 2023 Jan 16. Clin Microbiol Rev. 2023. PMID: 36645300 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

