Modeling Aβ42 Accumulation in Response to Herpes Simplex Virus 1 Infection: 2D or 3D?
- PMID: 33268524
- PMCID: PMC8092822
- DOI: 10.1128/JVI.02219-20
Modeling Aβ42 Accumulation in Response to Herpes Simplex Virus 1 Infection: 2D or 3D?
Abstract
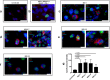
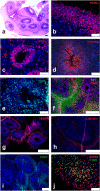
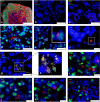
Alzheimer's disease is a progressive neurodegenerative disease characterized neuropathologically by presence of extracellular amyloid plaques composed of fibrillar amyloid beta (Aβ) peptides and intracellular neurofibrillary tangles. Post-mortem and in vivo studies implicate HSV-1 infection in the brain as a precipitating factor in disease/pathology initiation. HSV-1 infection of two-dimensional (2D) neuronal cultures causes intracellular accumulation of Aβ42 peptide, but these 2D models do not recapitulate the three-dimensional (3D) architecture of brain tissue.We employed human induced pluripotent stem cells (hiPSCs) to compare patterns of Aβ42 accumulation in HSV-1 infected 2D (neuronal monolayers) and 3D neuronal cultures (brain organoids). Akin to prior studies, HSV-1-infected 2D cultures showed Aβ42 immunoreactivity in cells expressing the HSV-1 antigen ICP4 (ICP4+). Conversely, accumulation of Aβ42 in ICP4+ cells in infected organoids was rarely observed. These results highlight the importance of considering 3D cultures to model host-pathogen interaction.IMPORTANCE The "pathogen" hypothesis of Alzheimer's disease (AD) proposes that brain HSV-1 infection could be an initial source of amyloid beta (Aβ) peptide-containing amyloid plaque development. Aβ accumulation was reported in HSV-1-infected 2D neuronal cultures and neural stem cell cultures, as well as in HSV-1-infected 3D neuronal culture models.The current study extends these findings by showing different patterns of Aβ42 accumulation following HSV-1 infection of 2D compared to 3D neuronal cultures (brain organoids). Specifically, 2D neuronal cultures showed Aβ42-immunoreactivity mainly in HSV-1-infected cells and only rarely in uninfected cells or infected cells exposed to antivirals. Conversely, 3D brain organoids showed accumulation of Aβ42 mainly in non-infected cells surrounding HSV-1-infected cells. We suggest that because brain organoids better recapitulate architectural features of a developing brain than 2D cultures, they may be a more suitable model to investigate the involvement of HSV-1 in the onset of AD pathology.
Copyright © 2020 Abrahamson et al.
Figures
References
-
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. 2014. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515:274–278. doi:10.1038/nature13800. - DOI - PMC - PubMed
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. 2011. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:280–292. doi:10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

