Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial
- PMID: 33268527
- PMCID: PMC7708829
- DOI: 10.1136/bmj.m4328
Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial
Abstract
Objective: To evaluate and compare benefits and harms of three biological treatments with different modes of action versus active conventional treatment in patients with early rheumatoid arthritis.
Design: Investigator initiated, randomised, open label, blinded assessor, multiarm, phase IV study.
Setting: Twenty nine rheumatology departments in Sweden, Denmark, Norway, Finland, the Netherlands, and Iceland between 2012 and 2018.
Participants: Patients aged 18 years and older with treatment naive rheumatoid arthritis, symptom duration less than 24 months, moderate to severe disease activity, and rheumatoid factor or anti-citrullinated protein antibody positivity, or increased C reactive protein.
Interventions: Randomised 1:1:1:1, stratified by country, sex, and anti-citrullinated protein antibody status. All participants started methotrexate combined with (a) active conventional treatment (either prednisolone tapered to 5 mg/day, or sulfasalazine combined with hydroxychloroquine and intra-articular corticosteroids), (b) certolizumab pegol, (c) abatacept, or (d) tocilizumab.
Main outcome measures: The primary outcome was adjusted clinical disease activity index remission (CDAI≤2.8) at 24 weeks with active conventional treatment as the reference. Key secondary outcomes and analyses included CDAI remission at 12 weeks and over time, other remission criteria, a non-inferiority analysis, and harms.
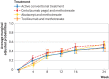
Results: 812 patients underwent randomisation. The mean age was 54.3 years (standard deviation 14.7) and 68.8% were women. Baseline disease activity score of 28 joints was 5.0 (standard deviation 1.1). Adjusted 24 week CDAI remission rates were 42.7% (95% confidence interval 36.1% to 49.3%) for active conventional treatment, 46.5% (39.9% to 53.1%) for certolizumab pegol, 52.0% (45.5% to 58.6%) for abatacept, and 42.1% (35.3% to 48.8%) for tocilizumab. Corresponding absolute differences were 3.9% (95% confidence interval -5.5% to 13.2%) for certolizumab pegol, 9.4% (0.1% to 18.7%) for abatacept, and -0.6% (-10.1% to 8.9%) for tocilizumab. Key secondary outcomes showed no major differences among the four treatments. Differences in CDAI remission rates for active conventional treatment versus certolizumab pegol and tocilizumab, but not abatacept, remained within the prespecified non-inferiority margin of 15% (per protocol population). The total number of serious adverse events was 13 (percentage of patients who experienced at least one event 5.6%) for active conventional treatment, 20 (8.4%) for certolizumab pegol, 10 (4.9%) for abatacept, and 10 (4.9%) for tocilizumab. Eleven patients treated with abatacept stopped treatment early compared with 20-23 patients in the other arms.
Conclusions: All four treatments achieved high remission rates. Higher CDAI remission rate was observed for abatacept versus active conventional treatment, but not for certolizumab pegol or tocilizumab versus active conventional treatment. Other remission rates were similar across treatments. Non-inferiority analysis indicated that active conventional treatment was non-inferior to certolizumab pegol and tocilizumab, but not to abatacept. The results highlight the efficacy and safety of active conventional treatment based on methotrexate combined with corticosteroids, with nominally better results for abatacept, in treatment naive early rheumatoid arthritis.
Trial registration: EudraCT2011-004720-35, NCT01491815.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from Academy of Finland, Finska Läkaresällskapet, South-Eastern Health Region (Norway), HUCH (Finland), Icelandic Society for Rheumatology, all health regions in Norway, NordForsk, Regionernes Medicinpulje (Denmark), Stockholm County Council (Sweden), Swedish Medical Research Council, Swedish Rheumatism Association, The Research Fund of University Hospital (Reykjavik, Iceland) for the submitted work; MLH reports grants from Nordforsk, from Danske Regioner during the conduct of the study; grants from Bristol-Myers Squibb, grants from AbbVie, grants from Roche, grants from Novartis, grants and personal fees from Merck, grants and personal fees from Biogen, grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees from Orion Pharma, personal fees from CellTrion, personal fees from Samsung Bioepsi, personal fees from Janssen Biologics BV, personal fees from MSD, outside the submitted work; she chairs the steering committee of the Danish Rheumatology Quality Registry (DANBIO), which receives public funding from the hospital owners and funding from pharamaceutical companies; EAH reports grants from NORDFORSK, grants from the Norwegian Regional Health Authorities, grants from the South-Eastern Norway Regional Health Authority, during the conduct of the study; personal fees from Pfizer, personal fees from AbbVie, personal fees from Celgene, personal fees from Novartis, personal fees from Janssen, personal fees from Gilead, personal fees from Eli-Lilly, personal fees from UCB, outside the submitted work; AR reports grants from the Swedish Research Council, financial support from AstraZeneca, outside the submitted work; DN reports grants from UCB, grants from BMS, during the conduct of the study; grants from AbbVie, grants from Celgene, grants from MSD, grants from Novartis, grants from Pfizer outside the submitted work; MN reports grants from BMS, during the conduct of the study; grants from Abbvie, grants from BMS, personal fees from Celltrion, grants from MSD, grants from Pfizer, personal fees from Eli Lilly, grants from Amgen, outside the submitted work; BG reports personal fees from Novartis, outside the submitted work; TU reports a grant from NORDFORSK during the conduct of the study; personal fees from Grünenthal, personal fees from Lilly, personal fees from Novartis, personal fees from Pfizer, outside the submitted work; MØ reports grants, personal fees and non-financial support from AbbVie, grants, personal fees and non-financial support from BMS, personal fees from Boehringer-Ingelheim, personal fees from Eli Lilly, personal fees and non-financial support from Janssen, grants, personal fees and non-financial support from Merck, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Roche, grants, personal fees and non-financial support from UCB, grants and personal fees from Celgene, personal fees from Sanofi, personal fees from Regeneron, grants, personal fees and non-financial support from Novartis, personal fees from Orion, personal fees from Hospira, outside the submitted work; MSH reports grants from the South-Eastern Norway Regional Health Authority, during the conduct of the study; personal fees from Lilly, outside the submitted work; SK reports receiving grants from AbbVie, MSD and Novartis outside the submitted work; AKHE reports receiving personal fees from AbbVie, personal fees from Pfizer, outside the submitted work; KLG reports grants from BMS, outside the submitted work; RT reports grants from Finnish Rheumatology Research Fund, during the conduct of the study; OH reports non-financial support from Pfizer, personal fees from Abbvie, personal fees from Novartis, during the conduct of the study; TSI reports non-financial support from DiaGraphIT, personal fees from Abbvie, personal fees from BMS, personal fees from Celgene, personal fees from Medac, personal fees from Merck, personal fees from Novartis, personal fees from Orion Pharma, personal fees from Pfizer, personal fees from Roche, personal fees from Sandoz, personal fees from UCB, personal fees from Bohringer Ingelheim, outside the submitted work; LU reports personal fees from Abbvie, Eli Lilly and Novartis (speaker fees), outside the submitted work; DJS reports grants from KLINBEFORSK, during the conduct of the study; TBL reports personal fees from UCB, outside the submitted work; GB reports personal fees from BMS, outside the submitted work; ABA reports personal fees from Abbvie, personal fees from Eli Lilly, personal fees from Novartis, personal fees from Pfizer, outside the submitted work; AB reports grants from BMS, during the conduct of the study; CT reports grants and personal fees from Bristol Myers-Squibb, personal fees from Roche, personal fees from Abbvie, personal fees from Pfizer, outside the submitted work; HR reports personal fees from MSD, personal fees from Roche, personal fees from Abbvie, personal fees from Celgene, outside the submitted work; JR reports grants from BMS, during the conduct of the study; JW reports fees from Celgene, fees from Eli Lilly, fees from Novartis, outside the submitted work; KM reports personal fees from Abbvie, personal fees from Celgene, personal fees from Medac, personal fees from BMS, outside the submitted work; OKS reports grants from the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding, during the conduct of the study; non-financial support from Pfizer, non-financial support from Novartis, non-financial support from MSD, personal fees from Boeringer Ingelheim, outside the submitted work; PP reports personal fees from Novartis Finland Oy, outside the submitted work; RØ reports personal fees from Bristol-Meyer Squibb, personal fees and non-financial support from AbbVie, personal fees from Gilead, personal fees from Janssen, personal fees from Eli-Lilly, personal fees from Novartis, outside the submitted work; SNC reports personal fees from Bristol Myers Squibb, personal fees from General Electric, outside the submitted work; SE reports personal fees from Novartis, outside the submitted work; TO reports personal fees from Eli Lilly, consultancy fee from Merck Sharp and Dohme, outside the submitted work; reports grants from BMS, during the conduct of the study; grants from Roche, grants from Mylan, other from Abbvie, outside the submitted work; VR reports grants from BMS, during the conduct of the study; grants from Roche, grants from Mylan, other from Abbvie, outside the submitted work; RvV reports grants from BMS, during the conduct of the study; grants from BMS, GSK, Lilly, UCB, grants from Pfizer, Roche, personal fees from AbbVie, AstraZeneca, Biogen, Biotest, Celgene, Galapagos, Gilead, Janssen, Pfizer, Servier, UCB, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016. http://onlinelibrary.wiley.com/doi/ - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
