Mossy Cells in the Dorsal and Ventral Dentate Gyrus Differ in Their Patterns of Axonal Projections
- PMID: 33268544
- PMCID: PMC7880284
- DOI: 10.1523/JNEUROSCI.2455-20.2020
Mossy Cells in the Dorsal and Ventral Dentate Gyrus Differ in Their Patterns of Axonal Projections
Abstract
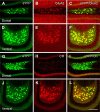
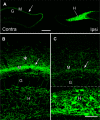
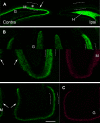
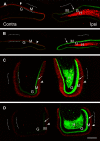
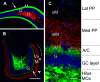
Mossy cells (MCs) of the dentate gyrus (DG) are a major group of excitatory hilar neurons that are important for regulating activity of dentate granule cells. MCs are particularly intriguing because of their extensive longitudinal connections within the DG. It has generally been assumed that MCs in the dorsal and ventral DG have similar patterns of termination in the inner one-third of the dentate molecular layer. Here, we demonstrate that axonal projections of MCs in these two regions are considerably different. MCs in dorsal and ventral regions were labeled selectively with Cre-dependent eYFP or mCherry, using two transgenic mouse lines (including both sexes) that express Cre-recombinase in MCs. At four to six weeks following unilateral labeling of MCs in the ventral DG, a dense band of fibers was present in the inner one-fourth of the molecular layer and extended bilaterally throughout the rostral-caudal extent of the DG, replicating the expected distribution of MC axons. In contrast, following labeling of MCs in the dorsal DG, the projections were more diffusely distributed. At the level of transfection, fibers were present in the inner molecular layer, but they progressively expanded into the middle molecular layer and, most ventrally, formed a distinct band in this region. Optical stimulation of these caudal fibers expressing ChR2 demonstrated robust EPSCs in ipsilateral granule cells and enhanced the effects of perforant path stimulation in the ventral DG. These findings suggest that MCs in the dorsal and ventral DG differ in the distribution of their axonal projections and possibly their function.SIGNIFICANCE STATEMENT Mossy cells (MCs), a major cell type in the hilus of the dentate gyrus (DG), are unique in providing extensive longitudinal and commissural projections throughout the DG. Although it has been assumed that all MCs have similar patterns of termination in the inner molecular layer of the DG, we discovered that the axonal projections of dorsal and ventral MCs differ. While ventral MC projections exhibit the classical pattern, with dense innervation in the inner molecular layer, dorsal MCs have a more diffuse distribution and expand into the middle molecular layer where they overlap and interact with innervation from the perforant path. These distinct locations and patterns of axonal projections suggest that dorsal and ventral MCs may have different functional roles.
Keywords: associational pathway; commissural pathway; dentate granule cells; hilus; hippocampus; mossy cells.
Copyright © 2021 the authors.
Figures




Similar articles
-
Dorsal and ventral mossy cells differ in their axonal projections throughout the dentate gyrus of the mouse hippocampus.Hippocampus. 2021 May;31(5):522-539. doi: 10.1002/hipo.23314. Epub 2021 Feb 18. Hippocampus. 2021. PMID: 33600026 Free PMC article.
-
Comparative Anatomy of the Dentate Mossy Cells in Nonhuman Primates: Their Spatial Distributions and Axonal Projections Compared With Mouse Mossy Cells.eNeuro. 2024 May 23;11(5):ENEURO.0151-24.2024. doi: 10.1523/ENEURO.0151-24.2024. Print 2024 May. eNeuro. 2024. PMID: 38688719 Free PMC article.
-
Alterations and Imbalance of Dorsal and Ventral Mossy Cells in a Mouse Model of Epilepsy.bioRxiv [Preprint]. 2024 Nov 7:2024.11.07.622490. doi: 10.1101/2024.11.07.622490. bioRxiv. 2024. PMID: 39574697 Free PMC article. Preprint.
-
Advances in understanding hilar mossy cells of the dentate gyrus.Cell Tissue Res. 2018 Sep;373(3):643-652. doi: 10.1007/s00441-017-2750-5. Epub 2017 Dec 8. Cell Tissue Res. 2018. PMID: 29222692 Free PMC article. Review.
-
The CA3 "backprojection" to the dentate gyrus.Prog Brain Res. 2007;163:627-37. doi: 10.1016/S0079-6123(07)63034-9. Prog Brain Res. 2007. PMID: 17765742 Free PMC article. Review.
Cited by
-
Dominant activities of fear engram cells in the dorsal dentate gyrus underlie fear generalization in mice.PLoS Biol. 2024 Jul 12;22(7):e3002679. doi: 10.1371/journal.pbio.3002679. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38995985 Free PMC article.
-
Alzheimer-like tau accumulation in dentate gyrus mossy cells induces spatial cognitive deficits by disrupting multiple memory-related signaling and inhibiting local neural circuit.Aging Cell. 2022 May;21(5):e13600. doi: 10.1111/acel.13600. Epub 2022 Mar 31. Aging Cell. 2022. PMID: 35355405 Free PMC article.
-
Adult Born Dentate Granule Cell Mediated Upregulation of Feedback Inhibition in a Mouse Model of Traumatic Brain Injury.J Neurosci. 2022 Sep 14;42(37):7077-7093. doi: 10.1523/JNEUROSCI.2263-21.2022. Epub 2022 Aug 24. J Neurosci. 2022. PMID: 36002261 Free PMC article.
-
Neuroprotective Effects of Lycium barbarum Berry on Neurobehavioral Changes and Neuronal Loss in the Hippocampus of Mice Exposed to Acute Ionizing Radiation.Dose Response. 2021 Nov 26;19(4):15593258211057768. doi: 10.1177/15593258211057768. eCollection 2021 Oct-Dec. Dose Response. 2021. PMID: 34887716 Free PMC article.
-
Alternatively spliced mini-exon B in PTPδ regulates excitatory synapses through cell-type-specific trans-synaptic PTPδ-IL1RAP interaction.Nat Commun. 2025 May 13;16(1):4415. doi: 10.1038/s41467-025-59685-3. Nat Commun. 2025. PMID: 40360498 Free PMC article.
References
-
- Bienkowski MS, Bowman I, Song MY, Gou L, Ard T, Cotter K, Zhu M, Benavidez NL, Yamashita S, Abu-Jaber J, Azam S, Lo D, Foster NN, Hintiryan H, Dong HW (2018) Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat Neurosci 21:1628–1643. 10.1038/s41593-018-0241-y - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
