Cocaine-Dependent Acquisition of Locomotor Sensitization and Conditioned Place Preference Requires D1 Dopaminergic Signaling through a Cyclic AMP, NCS-Rapgef2, ERK, and Egr-1/Zif268 Pathway
- PMID: 33268547
- PMCID: PMC7842751
- DOI: 10.1523/JNEUROSCI.1497-20.2020
Cocaine-Dependent Acquisition of Locomotor Sensitization and Conditioned Place Preference Requires D1 Dopaminergic Signaling through a Cyclic AMP, NCS-Rapgef2, ERK, and Egr-1/Zif268 Pathway
Abstract
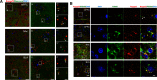
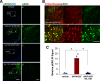
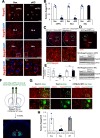
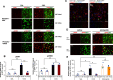
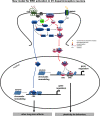
Elucidation of the mechanism of dopamine signaling to ERK that underlies plasticity in dopamine D1 receptor-expressing neurons leading to acquired cocaine preference is incomplete. NCS-Rapgef2 is a novel cAMP effector, expressed in neuronal and endocrine cells in adult mammals, that is required for D1 dopamine receptor-dependent ERK phosphorylation in mouse brain. In this report, we studied the effects of abrogating NCS-Rapgef2 expression on cAMP-dependent ERK→Egr-1/Zif268 signaling in cultured neuroendocrine cells; in D1 medium spiny neurons of NAc slices; and in either male or female mouse brain in a region-specific manner. NCS-Rapgef2 gene deletion in the NAc in adult mice, using adeno-associated virus-mediated expression of cre recombinase, eliminated cocaine-induced ERK phosphorylation and Egr-1/Zif268 upregulation in D1-medium spiny neurons and cocaine-induced behaviors, including locomotor sensitization and conditioned place preference. Abrogation of NCS-Rapgef2 gene expression in mPFC and BLA, by crossing mice bearing a floxed Rapgef2 allele with a cre mouse line driven by calcium/calmodulin-dependent kinase IIα promoter also eliminated cocaine-induced phospho-ERK activation and Egr-1/Zif268 induction, but without effect on the cocaine-induced behaviors. Our results indicate that NCS-Rapgef2 signaling to ERK in dopamine D1 receptor-expressing neurons in the NAc, but not in corticolimbic areas, contributes to cocaine-induced locomotor sensitization and conditioned place preference. Ablation of cocaine-dependent ERK activation by elimination of NCS-Rapgef2 occurred with no effect on phosphorylation of CREB in D1 dopaminoceptive neurons of NAc. This study reveals a new cAMP-dependent signaling pathway for cocaine-induced behavioral adaptations, mediated through NCS-Rapgef2/phospho-ERK activation, independently of PKA/CREB signaling.SIGNIFICANCE STATEMENT ERK phosphorylation in dopamine D1 receptor-expressing neurons exerts a pivotal role in psychostimulant-induced neuronal gene regulation and behavioral adaptation, including locomotor sensitization and drug preference in rodents. In this study, we examined the role of dopamine signaling through the D1 receptor via a novel pathway initiated through the cAMP-activated guanine nucleotide exchange factor NCS-Rapgef2 in mice. NCS-Rapgef2 in the NAc is required for activation of ERK and Egr-1/Zif268 in D1 dopaminoceptive neurons after acute cocaine administration, and subsequent enhanced locomotor response and drug seeking behavior after repeated cocaine administration. This novel component in dopamine signaling provides a potential new target for intervention in psychostimulant-shaped behaviors, and new understanding of how D1-medium spiny neurons encode the experience of psychomotor stimulant exposure.
Keywords: D1 dopaminergic signaling; Egr-1; MAPK; NCS-Rapgef2; cAMP; cocaine reward.
Copyright © 2021 the authors.
Figures




References
-
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685. 10.1523/JNEUROSCI.1039-08.2008 - DOI - PMC - PubMed
-
- Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J (2005) Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci 25:11444–11454. 10.1523/JNEUROSCI.1711-05.2005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
