Reprogramming to recover youthful epigenetic information and restore vision
- PMID: 33268865
- PMCID: PMC7752134
- DOI: 10.1038/s41586-020-2975-4
Reprogramming to recover youthful epigenetic information and restore vision
Abstract
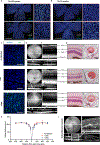
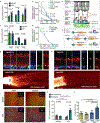
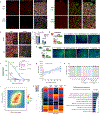
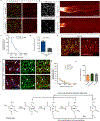
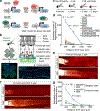
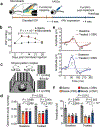
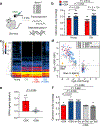
Ageing is a degenerative process that leads to tissue dysfunction and death. A proposed cause of ageing is the accumulation of epigenetic noise that disrupts gene expression patterns, leading to decreases in tissue function and regenerative capacity1-3. Changes to DNA methylation patterns over time form the basis of ageing clocks4, but whether older individuals retain the information needed to restore these patterns-and, if so, whether this could improve tissue function-is not known. Over time, the central nervous system (CNS) loses function and regenerative capacity5-7. Using the eye as a model CNS tissue, here we show that ectopic expression of Oct4 (also known as Pou5f1), Sox2 and Klf4 genes (OSK) in mouse retinal ganglion cells restores youthful DNA methylation patterns and transcriptomes, promotes axon regeneration after injury, and reverses vision loss in a mouse model of glaucoma and in aged mice. The beneficial effects of OSK-induced reprogramming in axon regeneration and vision require the DNA demethylases TET1 and TET2. These data indicate that mammalian tissues retain a record of youthful epigenetic information-encoded in part by DNA methylation-that can be accessed to improve tissue function and promote regeneration in vivo.
Conflict of interest statement
Conflict of interest
D.A.S. is a consultant to, inventor of patents licensed to, board member of and equity owner of Iduna Therapeutics, a Life Biosciences company developing epigenetic reprograming therapies. D.A.S. is an advisor to Zymo Research, an epigenetics tools company. Additional disclosures are at
Figures







Comment in
-
Sight restored by turning back the epigenetic clock.Nature. 2020 Dec;588(7836):34-36. doi: 10.1038/d41586-020-03119-1. Nature. 2020. PMID: 33268872 No abstract available.
-
Reversal of biological clock restores vision in old mice.Nature. 2020 Dec;588(7837):209. doi: 10.1038/d41586-020-03403-0. Nature. 2020. PMID: 33268879 No abstract available.
-
Hitting reverse on ageing.Nat Rev Genet. 2021 Feb;22(2):68-69. doi: 10.1038/s41576-020-00314-8. Nat Rev Genet. 2021. PMID: 33318705 No abstract available.
-
Retinal rejuvenation.Nat Rev Neurosci. 2021 Feb;22(2):73. doi: 10.1038/s41583-020-00422-8. Nat Rev Neurosci. 2021. PMID: 33324008 No abstract available.
-
Unlocking Tissue Regenerative Potential by Epigenetic Reprogramming.Cell Stem Cell. 2021 Jan 7;28(1):5-7. doi: 10.1016/j.stem.2020.12.006. Cell Stem Cell. 2021. PMID: 33417872
References
-
- Sinclair DA, Mills K & Guarente L Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science 277, 1313–1316 (1997). - PubMed
-
- Kennard MA Relation of age to motor impairment in man and in subhuman primates. Archives of Neurology & Psychiatry 44, 377–397, doi: 10.1001/archneurpsyc.1940.02280080137008 (1940). - DOI
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY021526/EY/NEI NIH HHS/United States
- R01 AG065403/AG/NIA NIH HHS/United States
- R24 EY028767/EY/NEI NIH HHS/United States
- R37 GM065204/GM/NIGMS NIH HHS/United States
- K99 AG068303/AG/NIA NIH HHS/United States
- R21 EY030276/EY/NEI NIH HHS/United States
- T32 AG023480/AG/NIA NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- R01 GM065204/GM/NIGMS NIH HHS/United States
- R01 EY026939/EY/NEI NIH HHS/United States
- P30 EY003790/EY/NEI NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States
- R01 AG067782/AG/NIA NIH HHS/United States
- 5P30EY012196/NH/NIH HHS/United States
- R01 EY025794/EY/NEI NIH HHS/United States
- P30 EY012196/EY/NEI NIH HHS/United States
- R37 AG028730/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

