Hippocampal Neural Stem Cell Grafting after Status Epilepticus Alleviates Chronic Epilepsy and Abnormal Plasticity, and Maintains Better Memory and Mood Function
- PMID: 33269095
- PMCID: PMC7673840
- DOI: 10.14336/AD.2020.1020
Hippocampal Neural Stem Cell Grafting after Status Epilepticus Alleviates Chronic Epilepsy and Abnormal Plasticity, and Maintains Better Memory and Mood Function
Abstract
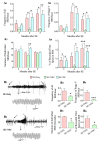
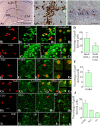
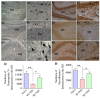
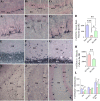
Hippocampal damage after status epilepticus (SE) leads to multiple epileptogenic changes, which lead to chronic temporal lobe epilepsy (TLE). Morbidities such as spontaneous recurrent seizures (SRS) and memory and mood impairments are seen in a significant fraction of SE survivors despite the administration of antiepileptic drugs after SE. We examined the efficacy of bilateral intra-hippocampal grafting of neural stem/progenitor cells (NSCs) derived from the embryonic day 19 rat hippocampi, six days after SE for restraining SE-induced SRS, memory, and mood impairments in the chronic phase. Grafting of NSCs curtailed the progression of SRS at 3-5 months post-SE and reduced the frequency and severity of SRS activity when examined at eight months post-SE. Reduced SRS activity was also associated with improved memory function. Graft-derived cells migrated into different hippocampal cell layers, differentiated into GABA-ergic interneurons, astrocytes, and oligodendrocytes. Significant percentages of graft-derived cells also expressed beneficial neurotrophic factors such as the fibroblast growth factor-2, brain-derived neurotrophic factor, insulin-like growth factor-1 and glial cell line-derived neurotrophic factor. NSC grafting protected neuropeptide Y- and parvalbumin-positive host interneurons, diminished the abnormal migration of newly born neurons, and rescued the reelin+ interneurons in the dentate gyrus. Besides, grafting led to the maintenance of a higher level of normal neurogenesis in the chronic phase after SE and diminished aberrant mossy fiber sprouting in the dentate gyrus. Thus, intrahippocampal grafting of hippocampal NSCs shortly after SE considerably curbed the progression of epileptogenic processes and SRS, which eventually resulted in less severe chronic epilepsy devoid of significant cognitive and mood impairments.
Keywords: EEG; cell transplantation; cognitive dysfunction; depression; hippocampal NSCs; memory; neural stem cells; neuroprotection; stem cell grafts; temporal lobe epilepsy.
copyright: © 2020 Hattiangady et al.
Figures




References
-
- Devinsky O (2004). Diagnosis and treatment of temporal lobe epilepsy. Rev Neurol Dis, 1:2-9. - PubMed
-
- Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS (2005). Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia, 46:1133-1139. - PubMed
-
- Mikulecka A, Druga R, Stuchlík A, Mareš P, Kubová H (2019). Comorbidities of early-onset temporal epilepsy: Cognitive, social, emotional, and morphologic dimensions. Exp Neurol, 320:113005. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
