The Critical Role of SIRT1 in Parkinson's Disease: Mechanism and Therapeutic Considerations
- PMID: 33269110
- PMCID: PMC7673849
- DOI: 10.14336/AD.2020.0216
The Critical Role of SIRT1 in Parkinson's Disease: Mechanism and Therapeutic Considerations
Abstract
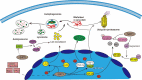
Silence information regulator 1 (SIRT1), a member of the sirtuin family, targets histones and many non-histone proteins and participates in various physiological functions. The enzymatic activity of SIRT1 is decreased in patients with Parkinson's disease (PD), which may reduce their ability to resist neuronal damage caused by various neurotoxins. As far as we know, SIRT1 can induce autophagy by regulating autophagy related proteins such as AMP-activated protein kinase, light chain 3, mammalian target of rapamycin, and forkhead transcription factor 1. Furthermore, SIRT1 can regulate mitochondrial function and inhibit oxidative stress mainly by maintaining peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) in a deacetylated state and thus maintaining a constant level of PGC-1α. Other studies have demonstrated that SIRT1 may play a role in the pathophysiology of PD by regulating neuroinflammation. SIRT1 deacetylases nuclear factor-kappa B and thus reduces its transcriptional activity, inhibits inducible nitric oxide synthase expression, and decreases tumor necrosis factor-alpha and interleukin-6 levels. SIRT1 can also upregulate heat shock protein 70 by deacetylating heat shock factor 1 to increase the degradation of α-synuclein oligomers. Few studies have focused on the relationship between SIRT1 single nucleotide polymorphisms and PD risk, so this topic requires further research. Based on the neuroprotective effects of SIRT1 on PD, many in vitro and in vivo experiments have demonstrated that some SIRT1 activators, notably resveratrol, have potential neuroprotective effects against dopaminergic neuronal damage caused by various neurotoxins. Thus, SIRT1 plays a critical role in PD development and might be a potential target for PD therapy.
Keywords: Parkinson's disease; SIRT1; STAC; neuroprotection; therapeutic potential.
copyright: © 2020 Li et al.
Conflict of interest statement
Competing interests The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson's disease.Biochim Biophys Acta. 2014 Jul;1842(7):902-15. doi: 10.1016/j.bbadis.2014.02.010. Epub 2014 Feb 25. Biochim Biophys Acta. 2014. PMID: 24582596
-
Treadmill Exercise Attenuates α-Synuclein Levels by Promoting Mitochondrial Function and Autophagy Possibly via SIRT1 in the Chronic MPTP/P-Induced Mouse Model of Parkinson's Disease.Neurotox Res. 2017 Oct;32(3):473-486. doi: 10.1007/s12640-017-9770-5. Epub 2017 Jun 27. Neurotox Res. 2017. PMID: 28656548
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
-
Resveratrol Reduces Oxidative Stress and Apoptosis in Podocytes via Sir2-Related Enzymes, Sirtuins1 (SIRT1)/Peroxisome Proliferator-Activated Receptor γ Co-Activator 1α (PGC-1α) Axis.Med Sci Monit. 2019 Feb 15;25:1220-1231. doi: 10.12659/MSM.911714. Med Sci Monit. 2019. PMID: 30765684 Free PMC article.
-
Natural products, PGC-1 α , and Duchenne muscular dystrophy.Acta Pharm Sin B. 2020 May;10(5):734-745. doi: 10.1016/j.apsb.2020.01.001. Epub 2020 Jan 8. Acta Pharm Sin B. 2020. PMID: 32528825 Free PMC article. Review.
Cited by
-
Understanding the Involvement of microRNAs in Mitochondrial Dysfunction and Their Role as Potential Biomarkers and Therapeutic Targets in Parkinson's Disease.J Alzheimers Dis. 2023;94(s1):S187-S202. doi: 10.3233/JAD-220449. J Alzheimers Dis. 2023. PMID: 35848027 Free PMC article. Review.
-
Mitochondria in health, disease, and aging.Physiol Rev. 2023 Oct 1;103(4):2349-2422. doi: 10.1152/physrev.00058.2021. Epub 2023 Apr 6. Physiol Rev. 2023. PMID: 37021870 Free PMC article. Review.
-
AMPK Signaling Pathway as a Potential Therapeutic Target for Parkinson's Disease.Adv Pharm Bull. 2024 Mar;14(1):120-131. doi: 10.34172/apb.2024.013. Epub 2023 Oct 14. Adv Pharm Bull. 2024. PMID: 38585465 Free PMC article. Review.
-
Expression levels of protein inhibitor of activated STAT (PIAS) family genes in Parkinson's disease patients: results from a case-control study.Acta Neurol Belg. 2025 Jun;125(3):727-735. doi: 10.1007/s13760-025-02752-9. Epub 2025 Feb 28. Acta Neurol Belg. 2025. PMID: 40016540
-
Resveratrol-Mediated Regulation of Mitochondria Biogenesis-associated Pathways in Neurodegenerative Diseases: Molecular Insights and Potential Therapeutic Applications.Curr Neuropharmacol. 2023;21(5):1184-1201. doi: 10.2174/1570159X20666221012122855. Curr Neuropharmacol. 2023. PMID: 36237161 Free PMC article. Review.
References
-
- Maloney EM, Djamshidian A, O'Sullivan SS (2017). Phenomenology and epidemiology of impulsive-compulsive behaviours in Parkinson's disease, atypical Parkinsonian disorders and non-Parkinsonian populations. J Neurol Sci, 374:47-52. - PubMed
-
- Shekhar S, Yadav Y, Singh AP, Pradhan R, Desai GR, Dey AB, et al. (2018). Neuroprotection by ethanolic extract of Syzygium aromaticum in Alzheimer's disease like pathology via maintaining oxidative balance through SIRT1 pathway. Exp Gerontol, 110:277-283. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
