Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: Four case reports
- PMID: 33269265
- PMCID: PMC7674713
- DOI: 10.12998/wjcc.v8.i21.5320
Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: Four case reports
Abstract
Background: Nafamostat mesylate (NM) may prove to be one of the key drugs effective against coronavirus disease 2019 (COVID-19) because of its anti-viral properties and the potential to manage coagulopathy. However, NM tends to increase serum potassium levels.
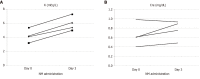
Case summary: We observed hyperkalemia immediately after NM administration (200 mg/d) in four consecutive patients who were admitted to the Kanazawa University Hospital with severe COVID-19 pneumonia. Urinary potassium excretion decreased after NM administration in three patients who underwent urinalysis.
Conclusion: NM is likely to produce hyperkalemia in patients with COVID-19. Therefore, it is necessary to monitor serum potassium values closely after NM initiation in COVID-19 patients who need respiratory support.
Keywords: COVID-19; Case report; Disseminated intravascular coagulation; Hyperkalemia; Nafamostat; Respiratory insufficiency.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no competing interests.
Figures
Similar articles
-
Nafamostat Mesylate Monotherapy in Patients with Moderate COVID-19: a Single-Center, Retrospective Study.Jpn J Infect Dis. 2022 Sep 22;75(5):484-489. doi: 10.7883/yoken.JJID.2021.699. Epub 2022 Apr 28. Jpn J Infect Dis. 2022. PMID: 35491224
-
The Effectiveness and Safety of Nafamostat Mesylate in the Treatment of COVID-19: a Meta-Analysis.Jpn J Infect Dis. 2024 May 23;77(3):182-186. doi: 10.7883/yoken.JJID.2023.315. Epub 2024 Jan 31. Jpn J Infect Dis. 2024. PMID: 38296543
-
RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia.J Clin Med. 2023 Oct 19;12(20):6618. doi: 10.3390/jcm12206618. J Clin Med. 2023. PMID: 37892756 Free PMC article.
-
Mechanisms of hyperkalemia caused by nafamostat mesilate.Gen Pharmacol. 1995 Dec;26(8):1627-32. doi: 10.1016/0306-3623(95)00072-0. Gen Pharmacol. 1995. PMID: 8745149 Review.
-
Clinical efficacy of Nafamostat Mesylate in combination with Favipiravir for COVID-19 pneumonia treatment review article.Ann Med Surg (Lond). 2021 Aug;68:102560. doi: 10.1016/j.amsu.2021.102560. Epub 2021 Jul 14. Ann Med Surg (Lond). 2021. PMID: 34276987 Free PMC article. Review.
Cited by
-
Protective Role of a TMPRSS2 Variant on Severe COVID-19 Outcome in Young Males and Elderly Women.Genes (Basel). 2021 Apr 19;12(4):596. doi: 10.3390/genes12040596. Genes (Basel). 2021. PMID: 33921689 Free PMC article.
-
Untapping host-targeting cross-protective efficacy of anticoagulants against SARS-CoV-2.Pharmacol Ther. 2022 May;233:108027. doi: 10.1016/j.pharmthera.2021.108027. Epub 2021 Oct 28. Pharmacol Ther. 2022. PMID: 34718070 Free PMC article. Review.
-
Incidence and risk factors for hyperkalaemia in patients treated for COVID-19 with nafamostat mesylate.J Clin Pharm Ther. 2022 Jul;47(7):1070-1078. doi: 10.1111/jcpt.13646. Epub 2022 Mar 21. J Clin Pharm Ther. 2022. PMID: 35313385 Free PMC article.
-
Efficacy and safety of nafamostat mesilate anticoagulation in blood purification treatment of critically ill patients: a systematic review and meta-analysis.Ren Fail. 2022 Dec;44(1):1263-1279. doi: 10.1080/0886022X.2022.2105233. Ren Fail. 2022. PMID: 35930302 Free PMC article.
-
SARS-CoV-2 Antiviral Therapy.Clin Microbiol Rev. 2021 Dec 15;34(4):e0010921. doi: 10.1128/CMR.00109-21. Epub 2021 Jul 28. Clin Microbiol Rev. 2021. PMID: 34319150 Free PMC article. Review.
References
-
- Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, Kinoshita N, Ohmagari N, Gohda J, Semba K, Matsuda Z, Kawaguchi Y, Kawaoka Y, Inoue JI. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses. 2020;12 - PMC - PubMed
-
- Nishimura H, Yamaya M. A Synthetic Serine Protease Inhibitor, Nafamostat Mesilate, Is a Drug Potentially Applicable to the Treatment of Ebola Virus Disease. Tohoku J Exp Med. 2015;237:45–50. - PubMed
-
- Muto S, Imai M, Asano Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen Pharmacol. 1995;26:1627–1632. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

