Secukinumab Improves Patient Perception of Anxiety and Depression in Patients with Moderate to Severe Psoriasis: A Post hoc Analysis of the SUPREME Study
- PMID: 33269404
- PMCID: PMC9366680
- DOI: 10.2340/00015555-3712
Secukinumab Improves Patient Perception of Anxiety and Depression in Patients with Moderate to Severe Psoriasis: A Post hoc Analysis of the SUPREME Study
Abstract
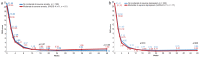
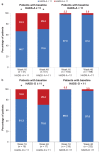
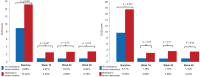
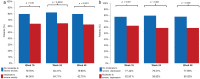
This study evaluated whether secukinumab treatment for patients with moderate to severe plaque psoriasis correlates with improvements in symptoms of anxiety and depression. SUPREME was a 24-week, phase IIIb, multicentre, prospective study conducted across 50 centres in Italy with an extension period of up to 72 weeks. Assessments used were: Psoriasis Area Sever-ity Index (PASI), Hospital Anxiety and Depression Scale (HADS) - Anxiety (HADS-A), and HADS - Depression (HADS-D) scores and Dermatology Quality Life Index (DLQI). Compared with baseline, a significantly greater proportion of patients who reported moderate to severe clinical symptoms of anxiety or depression (HADS-A or HADS-D ≥ 11) were free of moderate to severe symptoms at weeks 16 and 48. The PASI and DLQI scores reduced over time with secukinumab treatment. Psoriasis treatment with secukinumab for 48 weeks resulted in significantly improved skin clearance and a parallel improvement in symptoms of anxiety and depression, assessed by HADS.
Keywords: depression; psoriasis; quality of life; secukinumab; anxiety.
Conflict of interest statement
Figures
References
-
- Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271. - PubMed
-
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care 2016; 22: s216–224. - PubMed
-
- Wu JJ, Feldman SR, Koo J, Marangell LB. Epidemiology of mental health comorbidity in psoriasis. J Dermatolog Treat 2018; 29: 487–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

