Response-shift effects in neuromyelitis optica spectrum disorder: a secondary analysis of clinical trial data
- PMID: 33269417
- PMCID: PMC8068626
- DOI: 10.1007/s11136-020-02707-y
Response-shift effects in neuromyelitis optica spectrum disorder: a secondary analysis of clinical trial data
Abstract
Background: Researchers have long posited that response-shift effects may obfuscate treatment effects. The present work investigated possible response-shift effects in a recent clinical trial testing a new treatment for Neuromyelitis Optica Spectrum Disorder (NMOSD). This pivotal trial provided impressive support for the drug Eculizumab in preventing relapse, but less strong or null results as the indicators became more subjective or evaluative. This pattern of results suggests that response-shift effects are present.
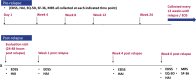
Methods: This secondary analysis utilized data from a randomized, double-blind trial evaluating the impact of Eculizumab in preventing relapses in 143 people with NMOSD. Treatment arm and then relapse status were hypothesized 'catalysts' of response shift in two series of analyses. We devised a "de-constructed" version of Oort structural-equation modeling using random-effects modeling for use in small samples. This method begins by testing an omnibus response-shift hypothesis and then, pending a positive result, implements a series of random-effects models to elucidate specific response-shift effects.
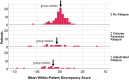
Results: In the omnibus test, the 'standard quality-of-life (QOL) model' captured substantially less well the experience of placebo as compared to Eculizumab group. Recalibration and reconceptualization response-shift effects were detected. Detected relapse-related response shifts included recalibration, reprioritization, and reconceptualization.
Conclusions: Trial patients experienced response shifts related to treatment- and relapse-related experiences. Published trial results likely under-estimated Eculizumab vs. Placebo differences due to recalibration and reconceptualization, and relapse effects due to recalibration, reprioritization, and reconceptualization. This novel random-effects- model application builds on response-shift theory and provides a small-sample method for better estimating treatment effects in clinical trials.
Keywords: Clinical trial; Clinician-assessed outcome; Definitive neuromyelitis optica; Neurologic; Neuromyelitis optica spectrum disorder; Patient-reported outcome; Response shift.
Conflict of interest statement
All authors declare that they have no potential conflicts of interest and report no disclosures.
Figures



Similar articles
-
Response-shift effects in neuromyelitis optica spectrum disorder: estimating response-shift-adjusted scores using equating.Qual Life Res. 2021 May;30(5):1283-1292. doi: 10.1007/s11136-020-02727-8. Epub 2021 Jan 5. Qual Life Res. 2021. PMID: 33398520 Free PMC article.
-
Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder.N Engl J Med. 2019 Aug 15;381(7):614-625. doi: 10.1056/NEJMoa1900866. Epub 2019 May 3. N Engl J Med. 2019. PMID: 31050279 Clinical Trial.
-
Benefits of eculizumab in AQP4+ neuromyelitis optica spectrum disorder: Subgroup analyses of the randomized controlled phase 3 PREVENT trial.Mult Scler Relat Disord. 2021 Jan;47:102641. doi: 10.1016/j.msard.2020.102641. Epub 2020 Nov 26. Mult Scler Relat Disord. 2021. PMID: 33310418 Clinical Trial.
-
Effectiveness of treatments in Neuromyelitis optica to modify the course of disease in adult patients. Systematic review of literature.Mult Scler Relat Disord. 2021 May;50:102869. doi: 10.1016/j.msard.2021.102869. Epub 2021 Feb 25. Mult Scler Relat Disord. 2021. PMID: 33711580
-
Eculizumab or ravulizumab treatment effect in people with neuromyelitis optica spectrum disorder: a plain language summary of three studies.J Comp Eff Res. 2025 Apr;14(4):e240177. doi: 10.57264/cer-2024-0177. Epub 2025 Feb 12. J Comp Eff Res. 2025. PMID: 39936529 Free PMC article. Review.
Cited by
-
Listening to the elephant in the room: response-shift effects in clinical trials research.J Patient Rep Outcomes. 2022 Sep 30;6(1):105. doi: 10.1186/s41687-022-00510-6. J Patient Rep Outcomes. 2022. PMID: 36178598 Free PMC article.
-
Double-blinded is not better than "mutually enlightened": implications of Lord-Besson et al.JNCI Cancer Spectr. 2023 Mar 1;7(2):pkad013. doi: 10.1093/jncics/pkad013. JNCI Cancer Spectr. 2023. PMID: 36790077 Free PMC article. No abstract available.
-
Long-COVID symptom burden and the experience of adversity: the importance of response-shift effects over 3 years of the pandemic.Qual Life Res. 2025 Aug;34(8):2233-2257. doi: 10.1007/s11136-025-03962-7. Epub 2025 Jun 6. Qual Life Res. 2025. PMID: 40478517
-
Response-shift effects in childhood cancer survivors: A prospective study.Psychooncology. 2023 Jul;32(7):1085-1095. doi: 10.1002/pon.6150. Epub 2023 May 15. Psychooncology. 2023. PMID: 37189277 Free PMC article.
References
-
- Schwartz CE, Sprangers MAG. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Social Science and Medicine. 1999;48(11):1531–1548. - PubMed
-
- Sprangers MAG, Schwartz CE. Integrating response shift into health-related quality of life research: A theoretical model. Social Science and Medicine. 1999;48(11):1507–1515. - PubMed
-
- Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MAG, Fayers PM. The clinical significance of adaptation to changing health: A meta-analysis of response shift. Quality of Life Research. 2006;15:1533–1550. - PubMed
-
- Sajobi TT, Brahmbatt R, Lix LM, Zumbo BD, Sawatzky R. Scoping review of response shift methods: current reporting practices and recommendations. Quality of Life Research. 2018;27(5):1133–1146. - PubMed
-
- Schwartz CE, Stucky BD, Rivers CS, Noonan VK, Finkelstein JA. Quality of life and adaptation in people with spinal cord injury: Response shift effects five-years post-injury. Archives of Physical Medicine and Rehabilitation. 2018;99:1599–1608. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

