Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes
- PMID: 33269554
- PMCID: PMC8049051
- DOI: 10.1111/dom.14277
Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes
Abstract
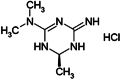
Imeglimin is an investigational first-in-class novel oral agent for the treatment of type 2 diabetes (T2D). Several pivotal phase III trials have been completed with evidence of statistically significant glucose lowering and a generally favourable safety and tolerability profile, including the lack of severe hypoglycaemia. Imeglimin's mechanism of action involves dual effects: (a) amplification of glucose-stimulated insulin secretion (GSIS) and preservation of β-cell mass; and (b) enhanced insulin action, including the potential for inhibition of hepatic glucose output and improvement in insulin signalling in both liver and skeletal muscle. At a cellular and molecular level, Imeglimin's underlying mechanism may involve correction of mitochondrial dysfunction, a common underlying element of T2D pathogenesis. It has been observed to rebalance respiratory chain activity (partial inhibition of Complex I and correction of deficient Complex III activity), resulting in reduced reactive oxygen species formation (decreasing oxidative stress) and prevention of mitochondrial permeability transition pore opening (implicated in preventing cell death). In islets derived from diseased rodents with T2D, Imeglimin also enhances glucose-stimulated ATP generation and induces the synthesis of nicotinamide adenine dinucleotide (NAD+ ) via the 'salvage pathway'. In addition to playing a key role as a mitochondrial co-factor, NAD+ metabolites may contribute to the increase in GSIS (via enhanced Ca++ mobilization). Imeglimin has also been shown to preserve β-cell mass in rodents with T2D. Overall, Imeglimin appears to target a key root cause of T2D: defective cellular energy metabolism. This potential mode of action is unique and has been shown to differ from that of other major therapeutic classes, including biguanides, sulphonylureas and glucagon-like peptide-1 receptor agonists.
Keywords: Imeglimin, mechanism, mitochondria, therapeutic, type 2 diabetes.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
S.H.B., P.F., S.B. and D.E.M. are employees of Poxel SA and own stock in the company. M.K. and E.F. have nothing to declare. G.V. owns Poxel SA stock. A.L.B. has nothing to declare regarding the present work.
Figures

 ) or vehicle (0.5% methylcellulose; black bars
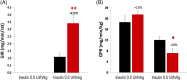
) or vehicle (0.5% methylcellulose; black bars  ) for 15 days. An euglycaemic hyperinsulinaemic clamp was then conducted, beginning 45 minutes after the last administration of Imeglimin or vehicle in overnight fasted rats (15 hours). Plasma insulin was increased to a constant level via primed, continuous infusion of exogenous insulin (0.5 UI/kg/h); plasma glucose was maintained at a constant euglycaemic level by varying the infusion of exogenous glucose. Two key variables were assessed during the clamp: A, The steady state glucose infusion rate (GIR) was measured as an index of whole body insulin sensitivity. B, [3‐3H]‐glucose was infused to assess endogenous glucose production (glucose production rate [GPR]). Both basal and hyperinsulinaemic conditions were studied. *P < .05; **P < .01 vs. vehicle control (Student t‐test); n = 10 rats per group
) for 15 days. An euglycaemic hyperinsulinaemic clamp was then conducted, beginning 45 minutes after the last administration of Imeglimin or vehicle in overnight fasted rats (15 hours). Plasma insulin was increased to a constant level via primed, continuous infusion of exogenous insulin (0.5 UI/kg/h); plasma glucose was maintained at a constant euglycaemic level by varying the infusion of exogenous glucose. Two key variables were assessed during the clamp: A, The steady state glucose infusion rate (GIR) was measured as an index of whole body insulin sensitivity. B, [3‐3H]‐glucose was infused to assess endogenous glucose production (glucose production rate [GPR]). Both basal and hyperinsulinaemic conditions were studied. *P < .05; **P < .01 vs. vehicle control (Student t‐test); n = 10 rats per group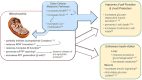
 Nicotinamide Phosphoribosyl‐transferase;
Nicotinamide Phosphoribosyl‐transferase;  Nicotinamide Adenine Dinucleotide (NAD+)
Nicotinamide Adenine Dinucleotide (NAD+)References
-
- Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab. 2012;14:852‐858. - PubMed
-
- Fouqueray P, Pirags V, Diamant M, et al. The efficacy and safety of imeglimin as add‐on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;37:1924‐1930. - PubMed
-
- Crabtree TSJ, DeFronzo RA, Ryder REJ, Bailey CJ. Imeglimin, a novel, first in‐class, blood glucose‐lowering agent: a systematic review and meta‐analysis of clinical evidence. Brit J Diabetes. 2020;20:28‐31.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

