Entacapone promotes hippocampal neurogenesis in mice
- PMID: 33269743
- PMCID: PMC8224137
- DOI: 10.4103/1673-5374.300447
Entacapone promotes hippocampal neurogenesis in mice
Abstract
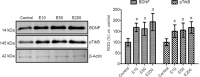
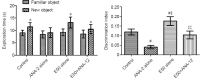
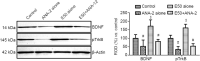
Entacapone, a catechol-O-methyltransferase inhibitor, can strengthen the therapeutic effects of levodopa on the treatment of Parkinson's disease. However, few studies are reported on whether entacapone can affect hippocampal neurogenesis in mice. To investigate the effects of entacapone, a modulator of dopamine, on proliferating cells and immature neurons in the mouse hippocampal dentate gyrus, 60 mice (7 weeks old) were randomly divided into a vehicle-treated group and the groups treated with 10, 50, or 200 mg/kg entacapone. The results showed that 50 and 200 mg/kg entacapone increased the exploration time for novel object recognition. Immunohistochemical staining results revealed that after entacapone treatment, the numbers of Ki67-positive proliferating cells, doublecortin-positive immature neurons, and phosphorylated cAMP response element-binding protein (pCREB)-positive cells were significantly increased. Western blot analysis results revealed that treatment with tyrosine kinase receptor B (TrkB) receptor antagonist significantly decreased the exploration time for novel object recognition and inhibited the expression of phosphorylated TrkB and brain-derived neurotrophic factor (BDNF). Entacapone treatment antagonized the effects of TrkB receptor antagonist. These results suggest that entacapone treatment promoted hippocampal neurogenesis and improved memory function through activating the BDNF-TrkB-pCREB pathway. This study was approved by the Institutional Animal Care and Use Committee of Seoul National University (approval No. SNU-130730-1) on February 24, 2014.
Keywords: brain-derived neurotrophic factor; entacapone; hippocampus; neurogenesis; neurotrophic factor; phosphorylated cAMP response element-binding protein; tyrosine kinase receptor B receptor.
Conflict of interest statement
None
Figures
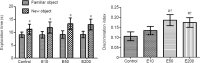



Similar articles
-
Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor, and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus.Brain Behav. 2019 Sep;9(9):e01388. doi: 10.1002/brb3.1388. Epub 2019 Aug 20. Brain Behav. 2019. PMID: 31429533 Free PMC article.
-
Mechanisms underpinning AMP-activated protein kinase-related effects on behavior and hippocampal neurogenesis in an animal model of depression.Neuropharmacology. 2019 May 15;150:121-133. doi: 10.1016/j.neuropharm.2019.03.026. Epub 2019 Mar 23. Neuropharmacology. 2019. PMID: 30914305
-
Phosphoglycerate Mutase 1 Promotes Cell Proliferation and Neuroblast Differentiation in the Dentate Gyrus by Facilitating the Phosphorylation of cAMP Response Element-Binding Protein.Neurochem Res. 2019 Feb;44(2):323-332. doi: 10.1007/s11064-018-2678-5. Epub 2018 Nov 20. Neurochem Res. 2019. PMID: 30460638
-
Entacapone: a catechol-O-methyltransferase inhibitor for the adjunctive treatment of Parkinson's disease.Clin Ther. 2001 Jun;23(6):802-32; discussion 771. doi: 10.1016/s0149-2918(01)80071-0. Clin Ther. 2001. PMID: 11440283 Review.
-
Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson's disease.Drugs. 2000 Jun;59(6):1233-50. doi: 10.2165/00003495-200059060-00004. Drugs. 2000. PMID: 10882160 Review.
Cited by
-
Activation of medial septum cholinergic neurons restores cognitive function in temporal lobe epilepsy.Neural Regen Res. 2023 Nov;18(11):2459-2465. doi: 10.4103/1673-5374.371369. Neural Regen Res. 2023. PMID: 37282477 Free PMC article.
-
Tat-heat shock protein 10 ameliorates age-related phenotypes by facilitating neuronal plasticity and reducing age-related genes in the hippocampus.Aging (Albany NY). 2023 Nov 25;15(22):12723-12737. doi: 10.18632/aging.205182. Epub 2023 Nov 25. Aging (Albany NY). 2023. PMID: 38011257 Free PMC article.
-
BDNF/TrkB activators in Parkinson's disease: A new therapeutic strategy.J Cell Mol Med. 2024 May;28(10):e18368. doi: 10.1111/jcmm.18368. J Cell Mol Med. 2024. PMID: 38752280 Free PMC article. Review.
-
Extracts from the Leaves of Cissus verticillata Ameliorate High-Fat Diet-Induced Memory Deficits in Mice.Plants (Basel). 2021 Aug 31;10(9):1814. doi: 10.3390/plants10091814. Plants (Basel). 2021. PMID: 34579347 Free PMC article.
-
Research progress on hippocampal neurogenesis in autism spectrum disorder.Pediatr Investig. 2024 Jul 23;8(3):215-223. doi: 10.1002/ped4.12440. eCollection 2024 Sep. Pediatr Investig. 2024. PMID: 39347523 Free PMC article. Review.
References
-
- Blank M, Petry FS, Lichtenfels M, Valiati FE, Dornelles AS, Roesler R. TrkB blockade in the hippocampus after training or retrieval impairs memory: protection from consolidation impairment by histone deacetylase inhibition. J Neural Transm (Vienna) 2016;123:159–165. - PubMed
-
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HH. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. - PubMed
-
- Burgess NE, Jeffery KJ, O’Keefe JE. Oxford: Oxford University Press; 1999. The hippocampal and parietal foundations of spatial cognition.
LinkOut - more resources
Full Text Sources
Other Literature Sources

