Developmental origins of metabolic diseases
- PMID: 33270534
- PMCID: PMC8526339
- DOI: 10.1152/physrev.00002.2020
Developmental origins of metabolic diseases
Abstract
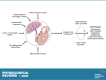
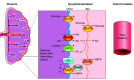
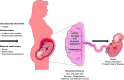
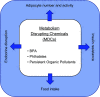
Almost 2 billion adults in the world are overweight, and more than half of them are classified as obese, while nearly one-third of children globally experience poor growth and development. Given the vast amount of knowledge that has been gleaned from decades of research on growth and development, a number of questions remain as to why the world is now in the midst of a global epidemic of obesity accompanied by the "double burden of malnutrition," where overweight coexists with underweight and micronutrient deficiencies. This challenge to the human condition can be attributed to nutritional and environmental exposures during pregnancy that may program a fetus to have a higher risk of chronic diseases in adulthood. To explore this concept, frequently called the developmental origins of health and disease (DOHaD), this review considers a host of factors and physiological mechanisms that drive a fetus or child toward a higher risk of obesity, fatty liver disease, hypertension, and/or type 2 diabetes (T2D). To that end, this review explores the epidemiology of DOHaD with discussions focused on adaptations to human energetics, placental development, dysmetabolism, and key environmental exposures that act to promote chronic diseases in adulthood. These areas are complementary and additive in understanding how providing the best conditions for optimal growth can create the best possible conditions for lifelong health. Moreover, understanding both physiological as well as epigenetic and molecular mechanisms for DOHaD is vital to most fully address the global issues of obesity and other chronic diseases.
Keywords: developmental origins; environmental toxins; metabolic diseases; obesity; placenta.
Conflict of interest statement
This work was supported by funding from the Robert Wood Johnson Foundation Grant 75084 (D. J. Hoffman); the National Institutes of Health Grants HD-068370 and HD-065007 (T.L. Powell), and P30 ES-005022 (E. S. Barrett); and the Canadian Institutes of Health Research Catalyst Grant CRU 1126 (D. B. Hardy).
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Centers for Disease Control and Prevention. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. Natl Health Stat Rep 122: 1–16, 2018. - PubMed
-
- Sanchez-Garrido MA, Ruiz-Pino F, Velasco I, Barroso A, Fernandois D, Heras V, Manfredi-Lozano M, Vazquez MJ, Castellano JM, Roa J, Pinilla L, Tena-Sempere M. Intergenerational influence of paternal obesity on metabolic and reproductive health parameters of the offspring: male-preferential impact and involvement of Kiss1-mediated pathways. Endocrinology 159: 1005–1018, 2018. doi: 10.1210/en.2017-00705. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

