Bisphosphonates or RANK-ligand-inhibitors for men with prostate cancer and bone metastases: a network meta-analysis
- PMID: 33270906
- PMCID: PMC8095056
- DOI: 10.1002/14651858.CD013020.pub2
Bisphosphonates or RANK-ligand-inhibitors for men with prostate cancer and bone metastases: a network meta-analysis
Abstract
Background: Different bone-modifying agents like bisphosphonates and receptor activator of nuclear factor-kappa B ligand (RANKL)-inhibitors are used as supportive treatment in men with prostate cancer and bone metastases to prevent skeletal-related events (SREs). SREs such as pathologic fractures, spinal cord compression, surgery and radiotherapy to the bone, and hypercalcemia lead to morbidity, a poor performance status, and impaired quality of life. Efficacy and acceptability of the bone-targeted therapy is therefore of high relevance. Until now recommendations in guidelines on which bone-modifying agents should be used are rare and inconsistent.
Objectives: To assess the effects of bisphosphonates and RANKL-inhibitors as supportive treatment for prostate cancer patients with bone metastases and to generate a clinically meaningful treatment ranking according to their safety and efficacy using network meta-analysis.
Search methods: We identified studies by electronically searching the bibliographic databases Cochrane Controlled Register of Trials (CENTRAL), MEDLINE, and Embase until 23 March 2020. We searched the Cochrane Library and various trial registries and screened abstracts of conference proceedings and reference lists of identified trials.
Selection criteria: We included randomized controlled trials comparing different bisphosphonates and RANKL-inihibitors with each other or against no further treatment or placebo for men with prostate cancer and bone metastases. We included men with castration-restrictive and castration-sensitive prostate cancer and conducted subgroup analyses according to this criteria.
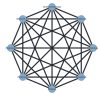
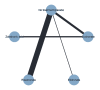
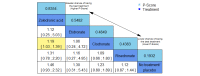
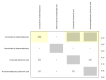
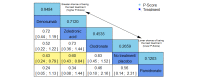
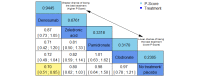
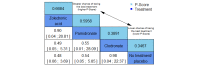
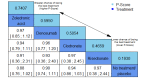
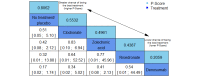
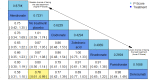
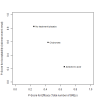
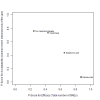
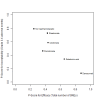
Data collection and analysis: Two review authors independently extracted data and assessed the quality of trials. We defined proportion of participants with pain response and the adverse events renal impairment and osteonecrosis of the jaw (ONJ) as the primary outcomes. Secondary outcomes were SREs in total and each separately (see above), mortality, quality of life, and further adverse events such as grade 3 to 4 adverse events, hypocalcemia, fatigue, diarrhea, and nausea. We conducted network meta-analysis and generated treatment rankings for all outcomes, except quality of life due to insufficient reporting on this outcome. We compiled ranking plots to compare single outcomes of efficacy against outcomes of acceptability of the bone-modifying agents. We assessed the certainty of the evidence for the main outcomes using the GRADE approach.
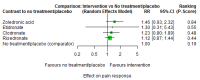
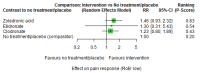
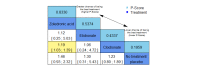
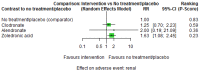
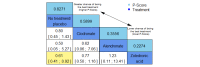
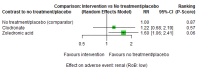
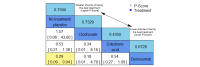
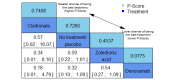
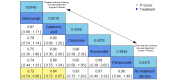
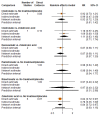
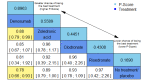
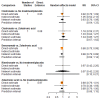
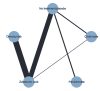
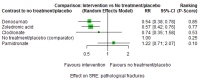
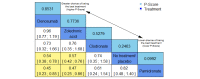
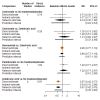
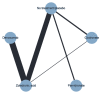
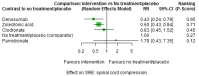
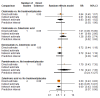
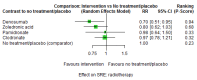
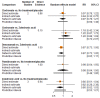
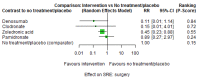
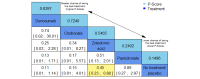
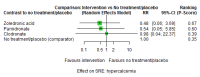
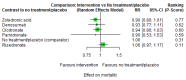
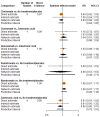
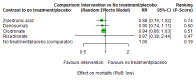
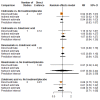
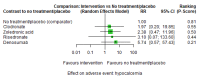
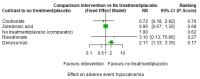
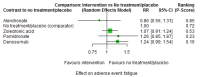
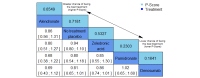
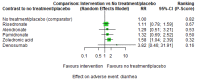
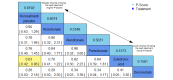
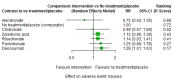
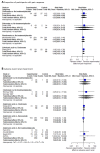
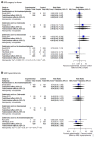
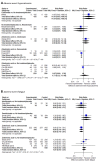
Main results: Twenty-five trials fulfilled our inclusion criteria. Twenty-one trials could be considered in the quantitative analysis, of which six bisphosphonates (zoledronic acid, risedronate, pamidronate, alendronate, etidronate, or clodronate) were compared with each other, the RANKL-inhibitor denosumab, or no treatment/placebo. By conducting network meta-analysis we were able to compare all of these reported agents directly and/or indirectly within the network for each outcome. In the abstract only the comparisons of zoledronic acid and denosumab against the main comparator (no treatment/placebo) are described for outcomes that were predefined as most relevant and that also appear in the 'Summary of findings' table. Other results, as well as results of subgroup analyses regarding castration status of participants, are displayed in the Results section of the full text. Treatment with zoledronic acid probably neither reduces nor increases the proportion of participants with pain response when compared to no treatment/placebo (risk ratio (RR) 1.46, 95% confidence interval (CI) 0.93 to 2.32; per 1000 participants 121 more (19 less to 349 more); moderate-certainty evidence; network based on 4 trials including 1013 participants). For this outcome none of the trials reported results for the comparison with denosumab. The adverse event renal impairment probably occurs more often when treated with zoledronic acid compared to treatment/placebo (RR 1.63, 95% CI 1.08 to 2.45; per 1000 participants 78 more (10 more to 180 more); moderate-certainty evidence; network based on 6 trials including 1769 participants). Results for denosumab could not be included for this outcome, since zero events cannot be considered in the network meta-analysis, therefore it does not appear in the ranking. Treatment with denosumab results in increased occurrence of the adverse event ONJ (RR 3.45, 95% CI 1.06 to 11.24; per 1000 participants 30 more (1 more to 125 more); high-certainty evidence; 4 trials, 3006 participants) compared to no treatment/placebo. When comparing zoledronic acid to no treatment/placebo, the confidence intervals include the possibility of benefit or harm, therefore treatment with zoledronic acid probably neither reduces nor increases ONJ (RR 1.88, 95% CI 0.73 to 4.87; per 1000 participants 11 more (3 less to 47 more); moderate-certainty evidence; network based on 4 trials including 3006 participants). Compared to no treatment/placebo, treatment with zoledronic acid (RR 0.84, 95% CI 0.72 to 0.97) and denosumab (RR 0.72, 95% CI 0.54 to 0.96) may result in a reduction of the total number of SREs (per 1000 participants 75 fewer (131 fewer to 14 fewer) and 131 fewer (215 fewer to 19 fewer); both low-certainty evidence; 12 trials, 5240 participants). Treatment with zoledronic acid and denosumab likely neither reduces nor increases mortality when compared to no treatment/placebo (zoledronic acid RR 0.90, 95% CI 0.80 to 1.01; per 1000 participants 48 fewer (97 fewer to 5 more); denosumab RR 0.93, 95% CI 0.77 to 1.11; per 1000 participants 34 fewer (111 fewer to 54 more); both moderate-certainty evidence; 13 trials, 5494 participants). Due to insufficient reporting, no network meta-analysis was possible for the outcome quality of life. One study with 1904 participants comparing zoledronic acid and denosumab showed that more zoledronic acid-treated participants than denosumab-treated participants experienced a greater than or equal to five-point decrease in Functional Assessment of Cancer Therapy-General total scores over a range of 18 months (average relative difference = 6.8%, range -9.4% to 14.6%) or worsening of cancer-related quality of life.
Authors' conclusions: When considering bone-modifying agents as supportive treatment, one has to balance between efficacy and acceptability. Results suggest that Zoledronic acid likely increases both the proportion of participants with pain response, and the proportion of participants experiencing adverse events However, more trials with head-to-head comparisons including all potential agents are needed to draw the whole picture and proof the results of this analysis.
Trial registration: ClinicalTrials.gov NCT00079001 NCT00003232 NCT00019695 NCT00104650 NCT00321620 NCT00216060 NCT00181558 NCT00268476 NCT00554918 NCT00685646.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Tina Jakob: none known
Yonas Mehari Tesfamariam: none known
Sascha Macherey: none known
Kathrin Kuhr: none known
Anne Adams: none known
Ina Monsef: none known
Axel Heidenreich: none known
Nicole Skoetz: none known
Figures











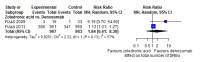







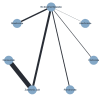
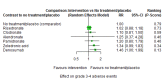
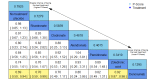

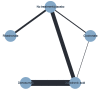







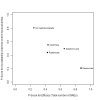





Update of
- doi: 10.1002/14651858.CD013020
Similar articles
-
Bone-modifying agents for reducing bone loss in women with early and locally advanced breast cancer: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jul 9;7(7):CD013451. doi: 10.1002/14651858.CD013451.pub2. Cochrane Database Syst Rev. 2024. PMID: 38979716 Free PMC article.
-
Bisphosphonates in multiple myeloma: an updated network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 18;12(12):CD003188. doi: 10.1002/14651858.CD003188.pub4. Cochrane Database Syst Rev. 2017. PMID: 29253322 Free PMC article.
-
Bisphosphonates and other bone agents for breast cancer.Cochrane Database Syst Rev. 2017 Oct 30;10(10):CD003474. doi: 10.1002/14651858.CD003474.pub4. Cochrane Database Syst Rev. 2017. PMID: 29082518 Free PMC article.
-
Bisphosphonates for advanced prostate cancer.Cochrane Database Syst Rev. 2017 Dec 26;12(12):CD006250. doi: 10.1002/14651858.CD006250.pub2. Cochrane Database Syst Rev. 2017. PMID: 29278410 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Bone Health Management in the Continuum of Prostate Cancer Disease.Cancers (Basel). 2022 Sep 2;14(17):4305. doi: 10.3390/cancers14174305. Cancers (Basel). 2022. PMID: 36077840 Free PMC article. Review.
-
Development of a core outcome set of clinical research on the integration of traditional Chinese and Western medicine for spinal metastases: a study protocol.BMJ Open. 2024 Sep 10;14(9):e083315. doi: 10.1136/bmjopen-2023-083315. BMJ Open. 2024. PMID: 39260838 Free PMC article.
-
Prostate cancer induced bone pain: pathobiology, current treatments and pain responses from recent clinical trials.Discov Oncol. 2022 Oct 18;13(1):108. doi: 10.1007/s12672-022-00569-z. Discov Oncol. 2022. PMID: 36258057 Free PMC article. Review.
-
Osteoclasts directly influence castration-resistant prostate cancer cells.Clin Exp Metastasis. 2022 Oct;39(5):801-814. doi: 10.1007/s10585-022-10179-2. Epub 2022 Aug 16. Clin Exp Metastasis. 2022. PMID: 35971022 Free PMC article.
-
Immunotherapy in the Fight Against Bone Metastases: A Review of Recent Developments and Challenges.Curr Treat Options Oncol. 2024 Nov;25(11):1374-1389. doi: 10.1007/s11864-024-01256-7. Epub 2024 Oct 22. Curr Treat Options Oncol. 2024. PMID: 39436492 Free PMC article. Review.
References
References to studies included in this review
Abetz 2006 {published data only}
-
- *Abetz L, Barghout V, Arbuckle R, Bosch V, Shirina N, Saad F. Impact of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases in a randomised placebo-control trial. Journal of Clinical Oncology 2006;24:4638.
-
- Barghout V, Abetz L, Arbuckle R, Bosch V, Hei Y, Saad F. Effect of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases based on performance status. Journal of Clinical Oncology - Supplement 2006;24(18):14544.
CALGB 90202 {published data only}
-
- Cook RJ, Coleman R, Brown J, Lipton A, Major P, Hei YJ, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clinical Cancer Research 2006;12:3361-7. - PubMed
-
- Smith MR. CALGB 90202: a randomized double-blind, placebo-controlled phase III study of early vs standard zoledronic acid to prevent skeletal-related events in men with prostate cancer metastatic to the bone. Clinical Advances in Hematology and Oncology 2006;4:897-8.
Elomaa 1992 {published data only}
-
- *Elomaa I, Kylmata T, Tammela T, Vitanen J, Ottelin M, Ruutu K, et al. Effect of oral clodronate on bone pain: a controlled study in patients with metastatic prostate cancer. International Journal of Urology and Nephrology 1992;24(2):159-66. - PubMed
-
- Taube T, Kylmala T, Lamberg-Allardt C, Tammela TLJ, Elomaa I. The effect of clodronate on bone in metastatic prostate cancer. Histomorphometric report of a double-blind randomised placebo-controlled study. European Journal of Cancer Part A: General Topics 1994;30:751-8. - PubMed
Ernst 2003 {published data only}
-
- *Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double-blind, controlled trial of mitoxantron/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. Journal of Clinical Oncology 2003;21(17):3335-42. - PubMed
Figg 2005 {published data only}
-
- *Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. Journal of Urology 2005;173:790-6. - PubMed
Fizazi 2009 {published data only}
-
- *Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology 2009;27:1564-71. - PubMed
-
- Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab in patients with bone metastases from castration-resistant prostate cancer and elevated bone resorption despite intravenous bisphosphonate (IV BP) therapy: analysis of a randomised phase II trial. Annals of Oncology 2008;19:viii153.
-
- Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. Journal of Urology 2009;182:509-16. - PubMed
-
- Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. Journal of Urology 2013;189:51-8. - PubMed
Fizazi 2011 {published data only}
-
- Brown J, Carducci M, Fizazi K, Smith M, Damião DR, Karsh L, et al. Denosumab versus zoledronic acid in patients with bone metastases from castration-resistant prostate cancer: results from a phase 3 randomized trial. Bone 2011;48(1):S16.
-
- Fizazi K, Brown JE, Carducci M, Shore ND, Sieber P, Kueppers F, et al. Denosumab in patients with metastatic prostate cancer previously treated with denosumab or zoledronic acid: 2-year open-label extension phase results from the pivotal phase 3 study. Annals of Oncology 2012;23:ix309.
-
- Fizazi K, Carducci MA, Smith MR, Damiao R, Brown JE, Karsh L, et al. A randomized phase III trial of denosumab versus zoledronic acid in patients with bone metastases from castration-resistant prostate cancer [abstract no. LBA4507]. Journal of Clinical Oncology 2010;28(18):951.
-
- Fizazi K, Massard C, Smith MR, Rader ME, Brown JE, Milecki P, et al. Baseline covariates impacting overall survival (OS) in a phase III study of men with bone metastases from castration-resistant prostate cancer. Journal of Clinical Oncology 2012;30:4642.
GU02‐4 {published data only}
-
- *Hahn NM, Yiannoutsos CT, Kirkpatrick K, Sharma J, Sweeney CJ. Failure to suppress markers of bone turnover on first-line hormone therapy for metastatic prostate cancer is associated with shorter time to skeletal-related event. Clinical Genitourinary Cancer 2014;12(1):33-40. - PubMed
-
- Sharma J, Yiannoutsos CT, Hahn NM, Sweeney C. Prognostic value of suppressed markers of bone turnover (BTO) after 6 months of androgen deprivation therapy (ADT) in prostate cancer. Journal of Clinical Oncology 2011;29:4594.
-
- Sweeney C, Dugan WM, Dreicer R, Chu F, Parks G, Baker K, et al. A randomized placebo-controlled trial of daily high-dose oral risedronate in men with metastatic prostate cancer commencing androgen deprivation therapy (ADT). Journal of Clinical Oncology 2010;28:e15000.
Kylmala 1993 {published data only}
-
- *Kylmala T, Tammela T, Risteli L, Risteli J, Taube T, Elomma I. Evaluation of the effect of oral clodronate on skeletal metastases with type I collagen metabolites. A controlled trial of the Finnish Prostate Cancer Group. European Journal of Cancer 1993;29(6):821-5. - PubMed
Kylmala 1997 {published data only}
Meulenbeld 2012 {published data only}22844568
-
- *Meulenbeld HJ, Werkhoven ED, Coenen JLLM, Creemers GJ, Loosveld OJL, Jong PC, et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic Castration Resistant Prostate Cancer (CRPC), the Netherlands Prostate Study (NePro). European Journal of Cancer 2012;48:2993-3000. - PubMed
Michaelson 2012 {published data only}
-
- *Michaelson MD, Kaufman DS, Kantoff P, Oh WK, Smith MR. Randomized phase II study of atrasentan alone or in combination with zoledronic acid in men with metastatic prostate cancer. Cancer 2012;107(3):530-5. - PubMed
Pan 2014 {published data only}
-
- *Pan Y, Jin H, Chen W, Yu Z, Ye T, Zheng Y, et al. Docetaxel with or without zoledronic acid for castration-resistant prostate cancer. International Urology and Nephrology 2014;46:2319-26. - PubMed
PR05 {published data only}38477744
-
- Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson ACR, et al. A double-blind, placebo-controlled, randomised trial of oral sodium clodronate for metastatic prostate cancer (MRC PRO5 Trial). Journal of the National Cancer Institute 2003;95(17):1300-11. - PubMed
Robertson 1995 {published data only}
-
- *Robertson AG, Reed NS, Ralston SH. Effect of oral clodronate on metastatic bone pain: a double-blind, placebo-controlled study. Journal of Clinical Oncology 1995;13(9):2427-30. - PubMed
Ryan 2007 {published data only}
-
- *Ryan CW, Huo D, Bylow K, Demers LM, Stadler WM, Henderson TO, et al. Suppression of bone density loss and bone turnover in patients with hormone-sensitive prostate cancer and receiving zoledronic acid. BJU International 2007;100:70-5. - PubMed
Saad 2010 {published data only}
-
- *Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 2010;76:1175-81. - PubMed
-
- Saad F, Chen YM, Gleason DM, Chin J. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clinical Genitourinary Cancer 2007;5:390-6. - PubMed
-
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. Journal of the National Cancer Institute 2004;96:879-82. - PubMed
-
- Saad F, Gleason DM, Murray R. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. Journal of the National Cancer Institute 2002;94(19):1458-68. - PubMed
-
- Saad F, Perez J, Cook R, Segal S. Evaluation of prostate-specific antigen kinetics during zoledronic acid therapy for bone metastases in patients with castration-resistant prostate cancer. Journal of Urology 2011;185:e288.
Small 2003 {published data only}
-
- *Small EJ, Matthew RS, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostatic cancer. Journal of Clinical Oncology 2003;21(23):4277-84. - PubMed
Smith 1989 {published data only}
-
- *Smith JAJ. Palliation of painful bone metastases from prostate cancer using sodium etidronate: results of a randomized, prospective, double-blind, placebo-controlled study. Journal of Urology 1989;141:85-7. - PubMed
STAMPEDE {published data only}
-
- *Mason MD, Clarke NW, James ND, Dearnaley DP, Spears MR, Ritchie AWS, et al. Adding celecoxib with or without zoledronic acid for hormone-naïve prostate cancer: long-term survival results from an adaptive, multiarm, multistage, platform, randomized controlled trial. Journal of Clinical Oncology 2017;35:1530-41. - PMC - PubMed
-
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Anderson J, et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer—A Multi-Arm Multi-Stage Randomised Controlled Trial. Clinical Oncology 2008;20:577-81. - PubMed
-
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163-77. - PMC - PubMed
-
- James ND, Sydes MR, Mason MD, Clarke NW, Anderson J, Dearnaley DP, et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from STAMPEDE (MRC PR08, CRUK/06/019), a randomized controlled trial. Journal of Clinical Oncology 2012;30:26. - PMC - PubMed
-
- James ND, Sydes MR, Mason MD, Clarke NW, Anderson J, Dearnaley DP, et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncology 2012;13:549-58. - PMC - PubMed
Strang 1997 {published data only}
-
- *Strang P, Nilsson S, Brandstedt S. The analgesic efficacy of clodronate compared with placebo inpatients with painful bone metastases from prostatic cancer. Anticancer Research 1997;17:4717-21. - PubMed
TRAPEZE 2016 {published data only}12808747
-
- *James N, Pirrie S, Pope A, Barton D, Andronis L, Goranitis I, et al. TRAPEZE: A randomised controlled trial of the clinical effectiveness and cost-effectiveness of chemotherapy with zoledronic acid, strontium-89, or both, in men with bony metastatic castration-refractory prostate cancer. Health Technology Assessment 2016;20(53):1-127. - PMC - PubMed
-
- James ND, Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, et al. Cost-effectiveness of zoledronic acid and strontium-89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate-refractory prostate cancer. (ISRCTN 12808747) TRAPEZE. Journal of Clinical Oncology 2015;33:e16108. - PubMed
-
- James ND, Pirrie S, Barton D, Brown JE, Billingham L, Collins SI, et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium-89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747). Journal of Clinical Oncology 2013;31(18):5000.
-
- James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncology 2016;2(4):493-9. - PubMed
-
- Porfiri E, Collins SI, Barton D, Billingham L, McLaren D, Nixon GG, et al. Initial feasibility and safety results from a phase II/III clinical trial to evaluate docetaxel (D) therapy in combination with zoledronic acid (ZA) {+/-} strontium-89 (Sr89) in hormone-refractory prostate cancer patients: ISRCTN12808747. Journal of Clinical Oncology 2010;28:4677.
Wang 2013 {published data only}
-
- *Wang F, Chen W, Chen H, Mo L, Jin H, Yu Z, et al. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Medical Oncology 2013;30:657. - PubMed
ZABTON‐PC {published data only}
-
- *Ueno S, Mizokami A, Fukagai T, Fujimoto N, Oh-Oka H, Kondo Y, et al. Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: the ZABTON-PC (zoledronic acid/androgen blockade trial on prostate cancer) study. Anticancer Research 2013;33:3837-44. - PubMed
ZAPCA {published data only}
-
- *Kamba T, Kamoto T, Maruo S, Kikuchi T, Shimizu Y, Namiki S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. International Journal of Clinical Oncology 2017;22:166-73. - PubMed
-
- Kamba T, Kamoto T, Shimizu Y, Namiki S, Fujimoto K, Kawanishi H, et al. A phase III, multicenter, randomized, controlled study of maximum androgen blockade with versus without zoledronic acid in treatment-naive prostate cancer patients with bone metastases: results of ZAPCA study. Journal of Clinical Oncology 2015;33:150. - PubMed
References to studies excluded from this review
Beer 2007 {published data only}
-
- Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT investigators. Journal of Clinical Oncology 2007;25(6):669-74. - PubMed
Body 2010 {published data only}
-
- Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. Journal of Bone and Mineral Research 2010;25(3):440-6. - PubMed
Brown 2011 {published data only}
-
- Brown JE, Barrios CH, Diel IJ, Facon T, Fizazi K, Ibrahim T, et al. Incidence and outcomes of osteonecrosis of the jaw from an integrated analysis of three pivotal randomized double-blind, double-dummy phase 3 trials comparing denosumab and zoledronic acid for treatment of bone metastases in advanced cancer patients or myeloma. Bone 2011;48(1):18-9.
Doria 2016 {published data only}
-
- Doria C, Leali PT, Solla F, Maestretti G, Balsano M, Scarpa RM. Denosumab is really effective in the treatment of osteoporosis secondary to hypogonadism in prostate carcinoma patients? A prospective randomized multicenter international study. Clinical Cases in Mineral and Bone Metabolism 2016;13(3):195-9. - PMC - PubMed
Doria 2017 {published data only}
-
- Doria C, Mosele GR, Solla F, Maestretti G, Balsano M, Scarpa RM. Treatment of osteoporosis secondary to hypogonadism in prostate cancer patients: a prospective randomized multicenter international study with denosumab vs. alendronate. Minerva Urologica e Nefrologica 2017;69(3):271-7. - PubMed
Heidenreich 2001 {published data only}
-
- Heidenreich A, Hofmann R, Engelmann UH. The use of bisphosphonate for the palliative treatment of painful bone metastasis due to hormone refractory prostate cancer. Journal of Urology 2001;165(1):136-40. - PubMed
Heidenreich 2002 {published data only}
-
- Heidenreich A, Elert A, Hofmann R. Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer and Prostatic Diseases 2002;5(3):231-5. - PubMed
Lang 2011 {published data only}
-
- Lang JM, Eickhoff JC, Binkley NC, Staab MJ, Liu G, Wilding G, et al. Randomized phase II trial evaluating different schedules of zoledronic acid administration on bone mineral density in patients with stage D prostate cancer beginning androgen deprivation. Journal of Clinical Oncology 2011;29(15):4643. - PMC - PubMed
Lang 2013 {published data only}
-
- Lang JM, Wallace M, Becker JT, Eickhoff JC, Buehring B, Binkley N, et al. A randomized phase ii trial evaluating different schedules of zoledronic acid on bone mineral density in patients with prostate cancer beginning androgen deprivation therapy. Clinical Genitourinary Cancer 2013;11(4):407-15. - PMC - PubMed
NTR503 {published data only}
-
- NTR503. Radiotherapy with or without ibandronate in the treatment of painful bone metastases of prostate cancer. trialregister.nl/trial/462 09.01.2006.
Patrick 2013 {published data only}
-
- Patrick D, Smith MR, Cleeland C, Fallowfield L, Tombal B, Oudard S, et al. The impact of bone metastases on pain: results from a phase III denosumab study in men with nonmetastatic castration-resistant prostate cancer. European Urology, Supplements 2013;12(1):e99-100.
Sawyer 1990 {published data only}
-
- Sawyer N, Newstead C, Drummond A, Cunningham J. Fast (4-h) or slow (24-h) infusions of pamidronate disodium (aminohydroxypropylidene diphosphonate (ADP)) as single shot treatment of hypercalcaemia. Bone and Mineral 1990;9(2):121-8. - PubMed
References to studies awaiting assessment
EUCTR2013‐001146‐34‐FR {published data only}
-
- EUCTR2013-001146-34-FR. Zoledronic acid in patients with metastatic prostate cancer treated with abiraterone acetate: impact on bone mineral density. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (first received 17 June 2015).
JPRN‐UMIN000002577 {published data only}
-
- JPRN-UMIN000002577. Effect of Zoledronic acid for Stage D2 hormone-sensitive prostate cancer patients. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000003142 05.10.2009.
JPRN‐UMIN000012967 {published data only}
-
- JPRN-UMIN000012967. Randomized control study to evaluate the efficacy of Denosumab versus zoledronic acid for treatment of bone metastases in men with prostate cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000015153 31.01.2014.
References to ongoing studies
NCT03336983 {published data only}
-
- NCT03336983. Luteinizing hormone-releasing hormone analogue and enzalutamide +/- zoledronic acid in prostate cancer patients. clinicaltrials.gov/show/nct03336983 08.11.2017.
Additional references
Alibhai 2017
-
- Alibhai S, Zukotynski K, Walker-Dilks C, Emmenegger U, Finelli A, Morgan S, et al. Bone Health and Bone-Targeted Therapies for Prostate Cancer. A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO). Available from https://www.cancercareontario.ca/en/content/bone-health-and-bone-targete... 2017. - PubMed
Anastasilakis 2009
-
- Anastasilakis AD, Toulis KA, Goulis DG, Polyzos SA, Delaroudis S, Giomisi A, et al. Efficacy and safety of denosumab in postmenopausal women with osteopenia or osteoporosis: a systematic review and a meta-analysis. Hormone and Metabolic Research 2009;41:721-9. - PubMed
Bartl 2007
-
- Bartl R, Frisch B, Tresckow E, Bartl C. Bisphosphonates in Medical Practice—Actions, Side Effects, Indications, Strategies. 1st edition. Dordrecht: Springer, 2007.
Bartl 2008
-
- Bartl R, Bartl C, Gradinger R. Use of bisphosphonates in orthopedic surgery [Einsatz der bisphosphonate in der orthopädie und unfallchirurgie]. Der Orthopäde 2008;37(6):595-614. - PubMed
Chaimani 2014
-
- Chaimani A, Salanti G, Becker L, Caldwell D, Higgins J, Li T. Protocol template for a Cochrane intervention review that compares multiple interventions. methods.cochrane.org/sites/methods.cochrane.org.cmi/files/public/uploads... (accessed prior to 18 July 2020).
Chaimani 2017
-
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. - PubMed
Clezardin 2013
Coleman 1997
-
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80(8 Suppl):1588-94. - PubMed
Coleman 2008
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. - PubMed
Dougall 2014
Egger 1997
Fitzpatrick 2014
-
- Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, et al. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. European Journal of Cancer 2014;50:1617-27. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. - PubMed
Gartrell 2014
Gartrell 2015
-
- Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. European Urology 2015;68(5):850-8. - PubMed
Gomez‐Veiga 2013
-
- Gomez-Veiga F, Ponce-Reixa J, Martinez-Breijo S, Planas J, Morote J. Advances in prevention and treatment of bone metastases in prostate cancer. Role of RANK/RANKL inhibition. Actas Urologicals Espanolas 2013;37(5):292-304. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT. Version accessed 21 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hanley 2012
Hegemann 2017
Hellstein 2011
-
- Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association 2011;142(11):1243-51. - PubMed
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011d
-
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howlader 2013
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al, National Cancer Institute. SEER Cancer Statistics Review, 1975-2010. www.seer.cancer.gov/csr/1975_2010 (accessed 23 June 2015).
Jin 2011
Krahn 2013
Lee 2014
-
- Lee SH, Chan RC, Chang SS, Tan YL, Chang KH, Lee MC, et al. Use of bisphosphonates and the risk of osteonecrosis among cancer patients: a systemic review and meta-analysis of the observational studies. Supportive Care in Cancer 2014;22(2):553-60. - PubMed
Macherey 2017
Mhaskar 2017
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. - PubMed
Mohler 2019
-
- Mohler JL, Antonarkis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2019;17(5):19-30. - PubMed
Mottet 2017
-
- Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. European Urology 2017;71(4):618-29. - PubMed
Netmeta 2017 [Computer program]
-
- Netmeta [Network Meta-Analysis using Frequentist Methods]. Rücker G, Schwarzer G, Krahn U, König J, Version R package version 0.9-7. CRAN.R-project.org/package=netmeta, 2017.
Neville‐Webbe 2010
-
- Neville-Webbe HL, Coleman RE. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. European Journal of Cancer 2010;46(7):1211-22. - PubMed
Nikolakopoulou 2020
O'Carrigan 2017
Oades 2002
-
- Oades GM, Coxon J, Colston KW. The potential role of bisphosphonates in prostate cancer. Prostate Cancer and Prostatic Diseases 2002;5(4):264-72. - PubMed
Parker 2015
-
- Parker C, Gillessen S, Heidenreich A, Horwich A, ESMO Guidelines Committee. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2015;26 Suppl 5:v69-77. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. - PubMed
Puhan 2014
-
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical research ed.) 2014;349:g5630. [PMID: ] - PubMed
Qi 2014
-
- Qi WX, Tang LN, He AN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomised controlled trials. International Journal of Clinical Oncology 2014;19(2):403-10. - PubMed
R 2017 [Computer program]
-
- R: A language and environment for statistical computing. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2017. Available at www.R-project.org.
Ramaswamy 2003
-
- Ramaswamy B, Shapiro CL. Bisphosphonates in the prevention and treatment of bone metastases. Oncology 2003;17:1261-70; discussion 1270-2, 1277-8, 1280. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2016
-
- Reyes C, Hitz M, Prieto-Alhambra D, Abrahamsen B. Risks and benefits of bisphosphonate therapies. Journal of Cellular Biochemistry 2016;117(1):20-8. - PubMed
Rücker 2012
-
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. [PMID: ] - PubMed
Rücker 2014
-
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. [PMID: ] - PubMed
Rücker 2015
Salanti 2014
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schwarzer 2015
-
- Schwarzer G, Carpenter JR, Rücker G. Chapter 8: Network meta-analysis. In: Meta-Analysis With R. Springer International Publishing Switzerland, 2015.
Smith 2009a
Sountoulides 2013
Soysa 2012
-
- Soysa NS, Alles N, Aoki K, Ohya K. Osteoclast formation and differentiation: an overview. Journal of Medical and Dental Sciences 2012;59(3):65-74. [PMID: ] - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematc Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tesfamariam 2019
-
- Tesfamariam Y, Jakob T, Wockel A, Adams A, Weigl A, Monsef I, et al. Adjuvant bisphosphonates or RANK-ligand inhibitors for patients with breast cancer and bone metastases: a systematic review and network meta-analysis. Critical Reviews in Oncology/Hematology 2019;137:1-8. - PubMed
Thobe 2011
References to other published versions of this review
Tesfamariam 2018
-
- Tesfamariam YM, Macherey S, Kuhr K, Becker I, Monsef I, Jakob T, et al. Bisphosphonates or RANK‐ligand‐inhibitors for men with prostate cancer and bone metastases: a Cochrane Review and network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD013020. [DOI: 10.1002/14651858.CD013020] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

